Abbreviations DSDSEDSO Appearance Colorless gas | Boiling point -15.2 °C | |
 | ||
Related compounds Thermodynamicdata Phase behavioursolid–liquid–gas | ||
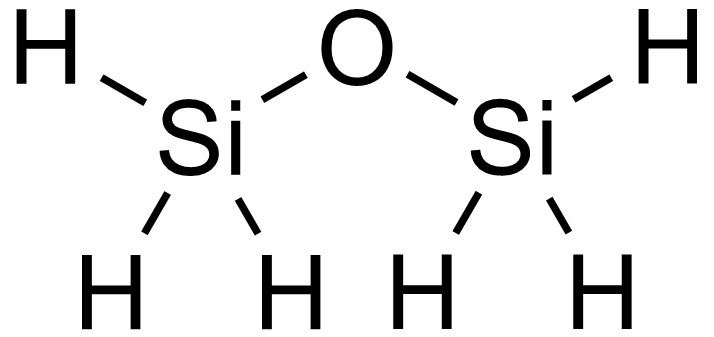
Disiloxane /ˈdaɪˈsaɪlɒkseɪn/ is a chemical compound with the chemical formula Si
2H
6O. It is the simplest siloxane, or oxasilane.
Contents
Properties and bonding
Disiloxane is a molecule with six equivalent Si-H bonds and two equivalent Si-O bonds.
At room temperature and standard pressure, disiloxane is a colourless, pungent gas. Disiloxane has a boiling point of −15 °C (5 °F) at a pressure of one atmosphere.
Production
Today, DSO is primarily produced by converting silane or silicon via gasification to a mixture of silicon monoxide, and hydrogen. This mixture is then converted into DSO in the presence of a catalyst. As described, this is a one-step (direct synthesis) process that permits both silanol synthesis and dehydration in the same process unit, with no silanol isolation and purification. Disiloxane reacts at low temperatures with aluminium halides to give the corresponding silyl and silylene halides and monosilane. Disiloxane is generally considered to be stable in water. It is more soluble than dimethyl ether. It hydrolyses very slowly:
H3SiOSiH3 + 3 H2O → 2 SiO2 + 6 H2Alternatively disiloxane can be prepared in the lab according to the following reactions:
H3SiX + H2O → H3SiOH + HX2 H3SiOH → H3SiOSiH3 + H2OUnlike dimethyl ether, it can be produced via autocondensation without a catalyst, as silanol is relatively unstable.
