Formula C2H6Hg Appearance Colorless liquid | Density 2.96 g/cm³ Classification Volatile organic compound | |
 | ||
Dimethylmercury ((CH3)2Hg) is an organomercury compound. This colorless liquid is one of the strongest known neurotoxins. It is described as having a slightly sweet smell, although inhaling enough vapor to detect its odor would be hazardous.
Contents
Synthesis, structure, reactions
The compound was one of the earliest organometallics reported, reflecting its considerable stability. It is formed by treating sodium amalgam with methyl halides:
Hg + 2 Na + 2 CH3I → Hg(CH3)2 + 2 NaIIt can also be obtained by alkylation of mercuric chloride with methyllithium:
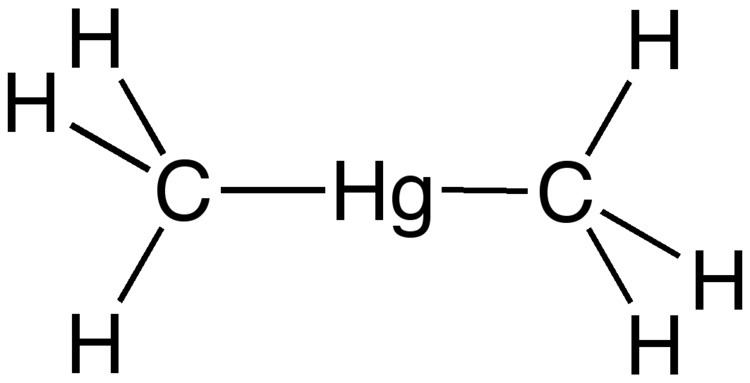
HgCl2 + 2 LiCH3 → Hg(CH3)2 + 2 LiClThe molecule adopts a linear structure with Hg-C bond lengths of 2.083 Å.
Reactions
The most striking feature of the compound is its nonreactivity toward water, whereas the corresponding organocadmium and organozinc compounds hydrolyze rapidly. The difference reflects the low affinity of Hg(II) for oxygen ligands. The compound reacts with mercuric chloride to give the mixed chloro-methyl compound:
(CH3)2Hg + HgCl2 → 2 CH3HgClWhereas dimethylmercury is a volatile liquid, CH3HgCl is a crystalline solid.
Use

Dimethylmercury currently has few applications because of the risks involved. As with many methyl-organometallics, it is a methylating agent that can donate its methyl groups to an organic molecule; however, the development of less acutely toxic nucleophiles such as dimethylzinc and trimethylaluminium, and the subsequent introduction of Grignard reagents (organometallic halides), has essentially rendered this compound obsolete in organic chemistry. It was formerly studied for reactions in which the methylmercury cation was bonded to the target molecule, forming potent bactericides; however, the bioaccumulation and ultimate toxicity of methylmercury has largely led it to be abandoned for this purpose in favor of the less toxic diethylmercury and ethylmercury compounds, which perform a similar function without the bioaccumulation hazard.
In toxicology, it was formerly used as a reference toxin. It has also been used to calibrate NMR instruments for detection of mercury, although diethylmercury and less toxic mercury salts are now preferred.
Safety
Dimethylmercury is extremely toxic and dangerous to handle. Absorption of doses as low as 0.1 mL can result in severe mercury poisoning. The risks are enhanced because of the high vapor pressure of the liquid.
Permeation tests showed that several types of disposable latex or polyvinyl chloride gloves (typically, about 0.1 mm thick), commonly used in most laboratories and clinical settings, had high and maximal rates of permeation by dimethylmercury within 15 seconds. The American Occupational Safety and Health Administration advises handling dimethylmercury with highly resistant laminated gloves with an additional pair of abrasion-resistant gloves worn over the laminate pair, and also recommends using a face shield and working in a fume hood.
Dimethylmercury crosses the blood–brain barrier easily, probably owing to formation of a complex with cysteine. It is eliminated from the organism slowly, and therefore has a tendency to bioaccumulate. The symptoms of poisoning may be delayed by months, resulting in many cases in which a diagnosis was ultimately discovered, but only at the point in which it is often too late for an effective treatment regimen to be successful.
The toxicity of dimethylmercury was highlighted with the death of the inorganic chemist Karen Wetterhahn of Dartmouth College in 1997. After spilling no more than a few drops of this compound on her latex-glove, the barrier was immediately compromised and within seconds it was absorbed into the back of her hand, quickly circulating and resulting in her death ten months later. This accident is a common toxicology case-study and spurred the development of modern advanced chemical-protection clothing which is now used when any exposure to severely toxic and/or highly penetrative substances is possible (e.g., in chemical munitions stockpiles and decontamination facilities).
