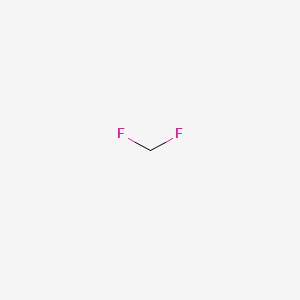Abbreviations HFC-32R-32FC-32 Density 1.1 g/cm³ | Formula CH2F2 Appearance Colorless gas | |
 | ||
Thermodynamicdata Phase behavioursolid–liquid–gas | ||
Difluoromethane, also called HFC-32 or R-32, is an organic compound of the dihalogenoalkane variety. It is based on methane, except that two of the four hydrogen atoms have been replaced by fluorine atoms, hence the formula is CH2F2 instead of CH4 for normal methane.

Uses

Difluoromethane is a refrigerant that has zero ozone depletion potential. Difluoromethane in a zeotropic (50%/50%) m/m mixture with pentafluoroethane (R-125) is known as R-410A, a common replacement for various chlorofluorocarbons (aka Freon) in new refrigerant systems, especially for air-conditioning. The zeotropic mix of difluoromethane with pentafluoroethane (R-125) and tetrafluoroethane (R-134a) is known as R-407A through R-407E depending on the composition. Likewise the azeotropic (48.2%/51.8% m/m) mixture with chlorotrifluoromethane (R13). As a refrigerant difluoromethane is classified as A2L - slightly flammable. Although it has zero ozone depletion potential, it has global warming potential 675 times that of carbon dioxide, based on a 100-year time frame.