Cytochromes c (cytC) are electron-transfer proteins having one or several heme c groups, bound to the protein by one or, more generally, two thioether bonds involving sulphydryl groups of cysteine residues. The fifth haem iron ligand is always provided by a histidine residue. Cytochromes c possess a wide range of properties and function in a large number of different redox processes. The founding member of this family is mitochondrial cytochrome c.
Ambler recognized four classes of cytC.
Class I includes the low-spin soluble cytC of mitochondria and bacteria, with the haem-attachment site towards the N-terminus, and the sixth ligand provided by a methionine residue about 40 residues further on towards the C-terminus. On the basis of sequence similarity, class I cytC were further subdivided into five classes, IA to IE. Class IB includes the eukaryotic mitochondrial cytC and prokaryotic 'short' cyt c2 exemplified by Rhodopila globiformis cyt c2; class IA includes 'long' cyt c2, such as Rhodospirillum rubrum cyt c2 and Aquaspirillum itersonii cytc-550, which have several extra loops by comparison with class IB cytC.
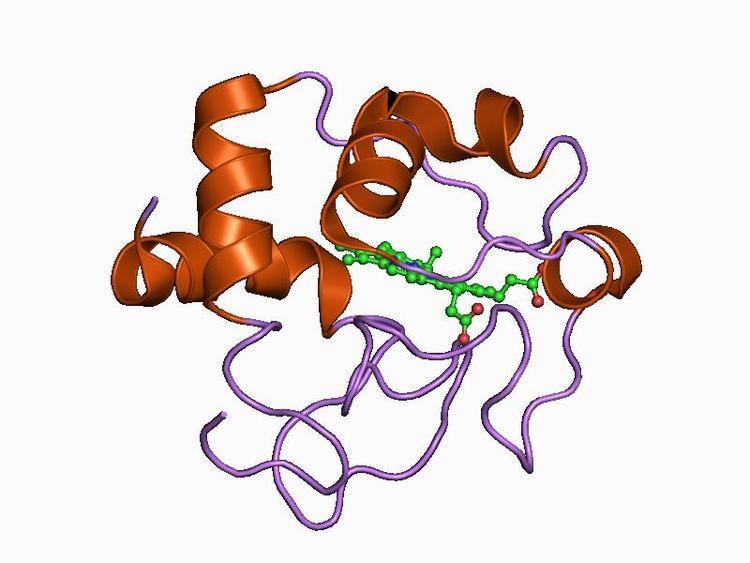
Class II includes the high-spin cytC' and a number of low-spin cytochromes, e.g. cyt c-556. The haem-attachment site is close to the C terminus. The cytC' are capable of binding such ligands as CO, NO or CN(-), albeit with rate and equilibrium constants 100 to 1,000,000-fold smaller than other high-spin haemoproteins. This, coupled with its relatively low redox potential, makes it unlikely that cytC' is a terminal oxidase. Thus cytC' probably functions as an electron transfer protein. The 3D structures of a number of cytC' have been determined. The molecule usually exists as a dimer, each monomer folding as a four-alpha-helix bundle incorporating a covalently-bound haem group at the core. The Chromatium vinosum cytC' exhibits dimer dissociation upon ligand binding.
Class III comprises the low redox potential multiple haem cytochromes: cyt C7 (trihaem), C3 (tetrahaem), and high-molecular-weight cytC, HMC (hexadecahaem), with only 30-40 residues per haem group. The haem c groups, all bis-histidinyl coordinated, are structurally and functionally nonequivalent and present different redox potentials in the range 0 to -400 mV. The 3D structures of a number of cyt C3 proteins have been determined. The proteins consist of 4-5 alpha-helices and 2 beta-strands wrapped around a compact core of four non-parallel haems, which present a relatively high degree of exposure to the solvent. The overall protein architecture, haem plane orientations and iron-iron distances are highly conserved.
Class IV includes complex proteins containing other prosthetic groups besides haem c, such as flavocytochromes c and cytochromes cd.