Molar mass 70.1 g/mol | Boiling point 49 °C Density 751 kg/m³ | |
 | ||
Related compounds | ||
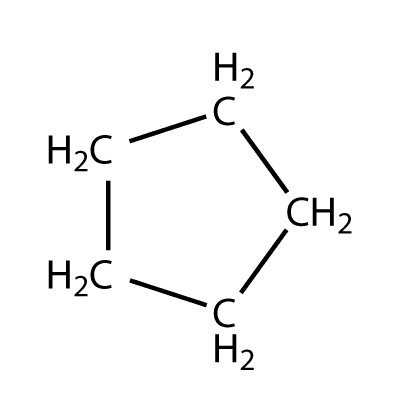
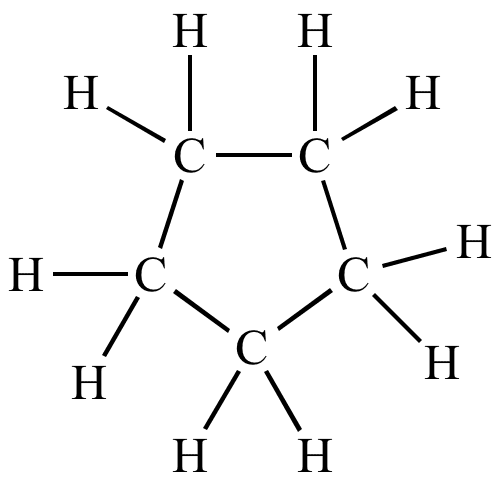
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula C5H10 and CAS Number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C and its boiling point is 49 °C. Cyclopentane is in the class of cycloalkanes, being alkanes that have one or more rings of carbon atoms.
Contents
It is formed by cracking cyclohexane in the presence of alumina at a high temperature and pressure.
It was first prepared in 1893 by the German chemist Johannes Wislicenus.
Industrial usage
Cyclopentane is used in the manufacture of synthetic resins and rubber adhesives and also as a blowing agent in the manufacture of polyurethane insulating foam, as found in many domestic appliances such as refrigerators and freezers, replacing environmentally damaging alternatives such as CFC-11 and HCFC-141b
Multiply alkylated cyclopentane (MAC) lubricants have low volatility and are used in some specialty applications.
The United States produces more than half a million kilograms of this chemical per year.
Formulation of cycloalkanes
Cycloalkanes can be formulated via a process known as catalytic reforming.

For example, 2-methylbutane can be reformed into cyclopentane, by use of a platinum catalyst. This is particularly well known in automobiles, as branched alkanes will burn much more readily.