Formula C6H10O2 Boiling point 241 °C Melting point -1 °C | Molar mass 114.14 g/mol Density 1.03 g/cm³ | |
 | ||
Poly caprolactone based nanoadjuvant video abstract 55892
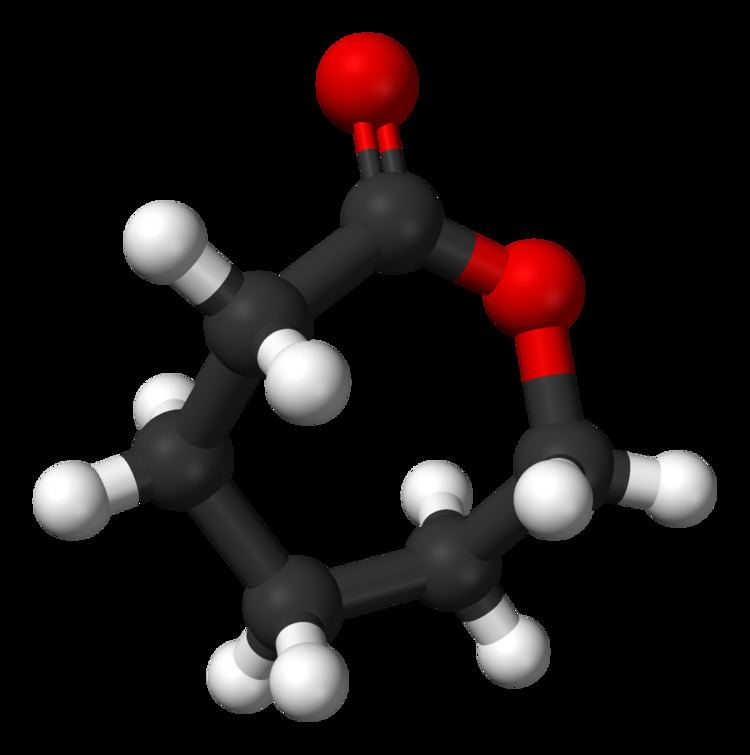
ε-Caprolactone or simply caprolactone is a cyclic ester, a member of the lactone family, with a seven-membered ring with the formula (CH2)5CO2. This colorless liquid is miscible with most organic solvents. It is produced on a large scale as a precursor to polycaprolactones.
Contents
- Poly caprolactone based nanoadjuvant video abstract 55892
- Non woven fibrous poly caprolactone using pmg
- Production and uses
- Reactions
- Related compounds
- Safety
- References
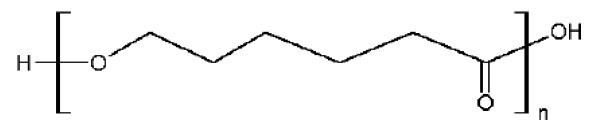
Non woven fibrous poly caprolactone using pmg
Production and uses

The great majority of caprolactone is consumed, often in situ, as a precursor to caprolactam. It is also a monomer used in the manufacture of highly specialised polymers. Ring-opening polymerization, for example, gives polycaprolactone. Another polymer is polyglecaprone, used as suture material in surgery.
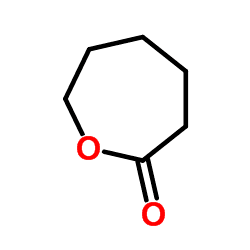
Caprolactone is prepared industrially by Baeyer-Villiger oxidation of cyclohexanone with peracetic acid. The three main manufacturers are BASF in the USA, Daicel in Japan and the largest Perstorp in Sweden.
Reactions
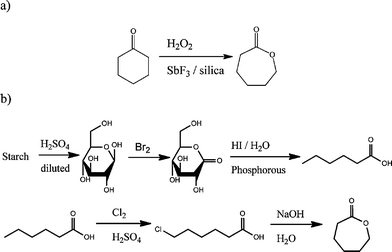
The major use of caprolactone is the production of polycaprolactones. These are split into two categories; the production of low molecular weight polycaprolactone polyols, used in specialty polyurethanes and coatings, and high molecular weight thermoplastics used in a variety of applications. There is published usage for caprolactone is its conversion to caprolactam, billions of kilograms of which are produced annually but not by this route.

Carbonylation of caprolactone gives, after hydrolysis, pimelic acid. The lactone ring is easily opened with nucleophiles including alcohols and water to give polylactones and eventually the 6-hydroxyadipic acid.
Related compounds
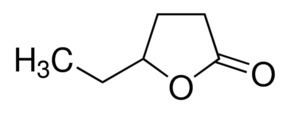
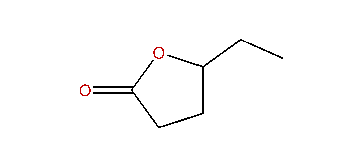
Several other caprolactones are known, although none approaches the technological importance of ε-caprolactone. These isomers include alpha-, beta-, gamma-, delta-caprolactones. All are chiral. Gamma-caprolactone is component of flower aromas and an insect pheromone. delta-Caprolactone is found in heated milk fat.
Safety
Caprolactone hydrolyses rapidly and the resulting hydroxycarboxylic acid displays unexceptional toxicity, as is common for the other hydroxycarboxylic acids. It is known to cause severe eye irritation. Exposure may result in corneal injury.