Formula Ca(HSO3)2 | Molar mass 202.22 g/mol | |
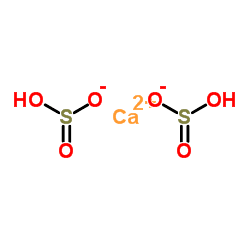 | ||
Calcium bisulfite (calcium bisulphite) is an inorganic compound which is the salt of a calcium cation and a bisulfite anion. It may be prepared by reacting lime with an excess of sulfurous acid, essentially a mixture of sulfur dioxide and water. It is a weak reducing agent, as is sulfur dioxide, sulfites, and any other compound containing sulfur in the +4 oxidation state. As a food additive it is used as a preservative under the E number E227. Calcium bisulfite is an acid salt and behaves like an acid in aqueous solution.
References
Calcium bisulfite Wikipedia(Text) CC BY-SA
