 | ||
Related compounds | ||
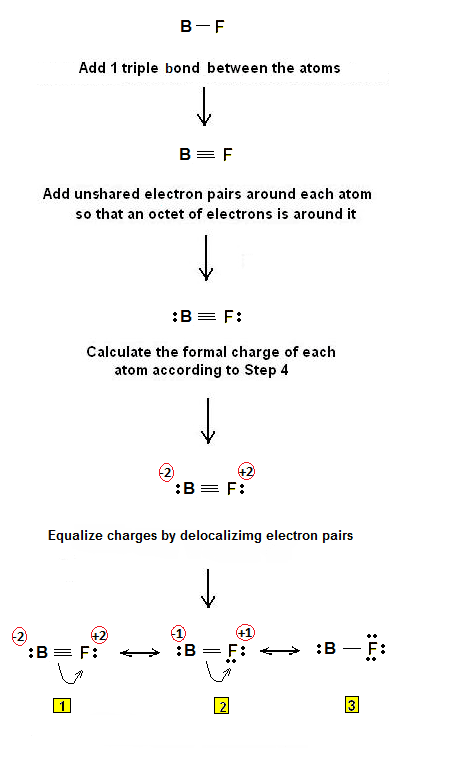
Boron monofluoride or fluoroborylene is a chemical compound with formula BF, one atom of boron and one of fluorine. It was discovered as an unstable gas and only in 2009 found to be a stable ligand combining with transition metals, in the same way as carbon monoxide. It is a subhalide, containing fewer than the normal number of fluorine atoms, compared with boron trifluoride. BF is isoelectronic with carbon monoxide and dinitrogen and each molecule has 14 electrons.
Contents
Structure
The experimental B–F bond length is 1.26267 Å. One reported computed bond order for the molecule is 1.4.
BF is unusual in that the dipole moment is inverted with fluorine having a positive charge even though it is the more electronegative element. This is explained by the 2sp orbitals of boron being reoriented and having a higher electron density. Backbonding, or the transfer of π orbital electrons for the fluorine atom is not required to explain the polarization.
Preparation
Boron monofluoride can be prepared by passing boron trifluoride gas at 2000 °C over a boron rod. It can be condensed at liquid nitrogen temperatures (−196 °C).
Properties
Boron monofluoride atoms have a dissociation energy of 7.8 eV or heat of formation −27.5±3 kcal/mole 760 kJ/mol. The first ionization potential is 11.115 eV. ωe is 1765 cm−1.
Reactions
BF can react with itself to form polymers of boron containing fluorine with between 10 and 14 boron atoms. BF reacts with BF3 to form B2F4. BF and B2F4 further combine to form B3F5. B3F5 is unstable above −50 °C and forms B8F12. This substance is a yellow oil.
BF reacts with acetylenes to make the 1,4-diboracyclohexadiene ring system. BF can condense with 2-butyne forming 1,4-difluoro-2,3,5,6-tetramethyl-1,4-diboracyclohexadiene. Also, it reacts with acetylene to make 1,4-difluoro-1,4-diboracyclohexadiene. Propene reacts to make a mix of cyclic and non-cyclic molecules which may contain BF or BF2.
BF hardly reacts with C2F4 or SiF4. BF does react with arsine, carbon monoxide, phosphorus trifluoride, phosphine and phosphorus trichloride to make adducts like (BF2)3B•AsH3, (BF2)3B•CO, (BF2)3B•PF3, (BF2)3B•PH3 and (BF2)3B•PCl3.
BF reacts with oxygen: BF + O2 → OBF + O ; with chlorine: BF + Cl2 → ClBF + Cl ; and with nitrogen dioxide BF + NO2 → OBF + NO.
Ligand
The first case of BF being a ligand on a transition element was demonstrated in 2009 with the compound (C5H5)2Ru2(CO)4(μ-BF). The BF was bound to both ruthenium atoms as a bridge.
Vidovic and Aldridge reacted NaRu(CO)2(C5H5) with (Et2O)·BF3. Note that the BF was formed in place rather than added on.
Earlier in 1968, K. Kämpfer, H. Nöth, W. Petz, and G. Schmid claimed that Fe(BF)(CO)4 was formed in the reaction of B2F4 with Fe(CO)5, however this has not been reproduced.
By reacting iron vapour with B2F4 and PF3 a substance with the formula (PF3)FeBF was produced. Hafnium, Thorium, Titanium and Zirconium can form a difluoride with a BF ligand at the low temperature of 6K. These come about by reacting the atomic metal with BF3.
BF is isoelectronic with carbon monoxide (CO) and so could form similar compounds to metal carbonyls. It is predicted to also bridge between two or three metal atoms (μ2 and μ3). Working with BF as a ligand is difficult due to its instability in the free state.
