Formula C4H8O3 Classification Hydroxy acids | Molar mass 104.1045 g/mol Appearance white solid | |
 | ||
Related compounds | ||
Beta hydroxybutyric acid
β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid, is an organic compound and a beta hydroxy acid with the formula CH3CH(OH)CH2CO2H; its conjugate base is beta-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound having two enantiomers, -β-hydroxybutyric acid and -β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature.
Contents
- Beta hydroxybutyric acid
- Biosynthesis
- Biological activity
- Laboratory and industrial chemistry
- References
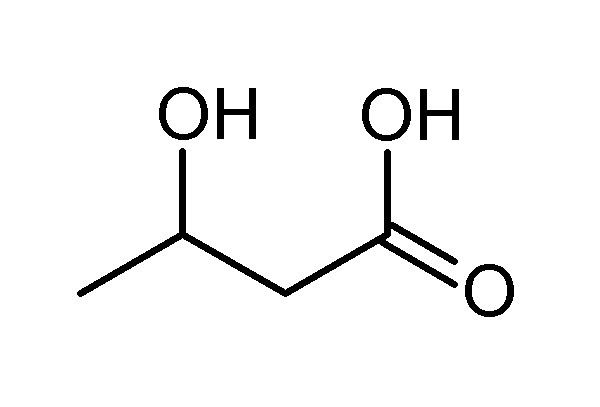
Biosynthesis
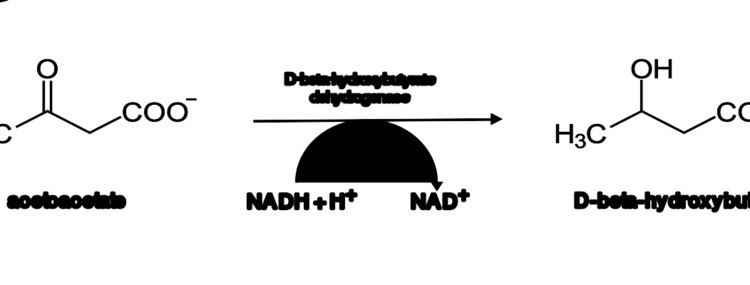
In humans, D-3-hydroxybutyrate is synthesized in the liver from acetoacetate, the first ketone produced in the fasting state. The biosynthesis is catalyzed by the enzyme beta-hydroxybutyrate dehydrogenase.
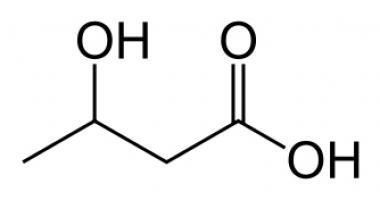
beta-hydroxybutyrate's concentration, as with other ketone bodies, is raised in ketosis. This elevated beta-hydroxybutyrate level is naturally expected, as beta-hydroxybutyrate is formed from acetoacetate. The compound can be used as an energy source by the brain when blood glucose is low. Diabetic patients can have their ketone levels tested via urine or blood to indicate diabetic ketoacidosis. In alcoholic ketoacidosis, this ketone body is produced in greatest concentration. Ketogenesis occurs if oxaloacetate in the liver cells is depleted, a circumstance created by reduced carbohydrate intake (through diet or starvation), prolonged, excessive alcohol consumption, and/or insulin deficiency. Because oxaloacetate is crucial for entry of Acetyl-CoA into the TCA cycle, the rapid production of Acetyl-CoA from fatty acid oxidation in the absence of ample oxaloacetate overwhelms the decreased capacity of the TCA cycle, and the resultant excess of Acetyl-CoA is shunted towards ketone body production.
Biological activity

β-Hydroxybutyric acid is able to cross the blood-brain-barrier into the central nervous system. Levels of β-hydroxybutyric acid increase in the liver, heart, muscle, brain, and other tissues with exercise, calorie restriction, fasting, and ketogenic diets. The compound has been found to act as a histone deacetylase (HDAC) inhibitor. Through inhibition of the HDAC class I isoenzymes HDAC2 and HDAC3, β-hydroxybutyric acid has been found to increase brain-derived neurotrophic factor (BNDF) levels and TrkB signaling in the hippocampus. Moreover, it has been determined that β-hydroxybutyric acid mediates the effects of exercise on BDNF levels in the hippocampus. These findings may have clinical relevance in the treatment of depression, anxiety, and cognitive impairment.

In epilepsy patients on the ketogenic diet, blood β-hydroxybutyrate levels correlate best with degree of seizure control. The threshold for optimal anticonvulsant effect appears to be approximately 4 mmol/L.
Laboratory and industrial chemistry
beta-Hydroxybutyric acid is the precursor to polyesters, which are biodegradable plastics. Known as poly(3-hydroxybutyrate), this polymer is also produced biologically by the bacteria Alcaligenes eutrophus.
beta-Hydroxybutyrate can be extracted from poly(3-hydroxybutyrate) by acid hydrolysis.
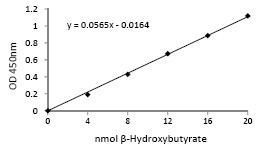
Blood beta-hydroxybutyrate levels are measured through a test that uses beta-hydroxybutyrate dehydrogenase, with NAD+ as an electron-accepting cofactor. The conversion of B-OHB to acetoacetate catalyzed by the enzyme reduces the NAD+ to NADH, generating an electrical change, the magnitude of which can then be used to extrapolate the amount of beta-hydroxybutyrate in the sample.
