Symbol Bet_v_I InterPro IPR000916 Pfam structures | Pfam PF00407 PROSITE PDOC00437 PDB RCSB PDB; PDBe; PDBj | |
 | ||
Bet v I allergen is a family of protein allergens. Allergies are hypersensitivity reactions of the immune system to specific substances called allergens (such as pollen, stings, drugs, or food) that, in most people, result in no symptoms.
Contents
Trees within the order Fagales possess particularly potent allergens, e.g. the prototypical Bet v1, the major white birch (Betula verrucosa) pollen antigen. Bet v 1 is the main cause of type I allergies observed in early spring. Type I, or immunoglobulin E-mediated (IgE-mediated) allergies affect 1 in 5 people in Europe and North America. Commonly observed symptoms are hay fever, dermatitis, asthma and, in severe cases, anaphylactic shock. First contact with these allergens results in sensitisation; subsequent contact produces a cross-linking reaction of IgE on mast cells and concomitant release of histamine. The inevitable symptoms of an allergic reaction ensue.
Categorization
A nomenclature system has been established for antigens (allergens) that cause IgE-mediated atopic allergies in humans. This nomenclature system is defined by a designation that is composed of the first three letters of the genus; a space; the first letter of the species name; a space and an Arabic number. In the event that two species names have identical designations, they are discriminated from one another by adding one or more letters (as necessary) to each species designation.
The allergens in this family include allergens with the following designations: Bet v 1, Dau c 1, and Pru a 1. Other proteins belonging to this family include the major pollen allergens:
Structure
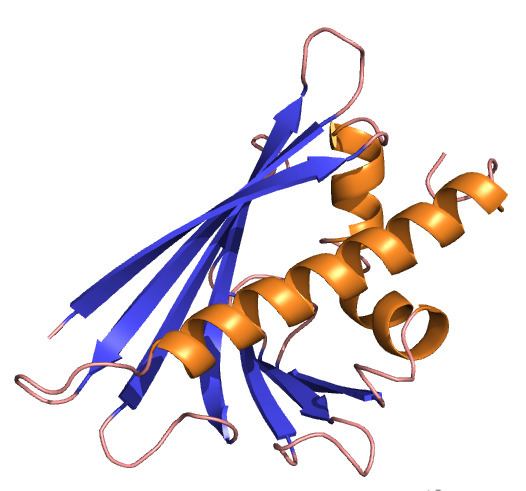
NMR analysis has confirmed earlier predictions of the protein structure and site of the major T-cell epitope. The Bet v 1 protein comprises 6 anti-parallel beta-strands and 3 alpha-helices. Four of the strands dominate the global fold, and 2 of the helices form a C-terminal amphipathic helical motif. This motif is believed to be the T-cell epitope. However, one very striking feature of the three-dimensional structure of Bet v 1 is the presence of a large hydrophobic cavity, which is open to the exterior and probably functions as a ligand binding site.
The motif is also found in:
Additionally, the core domain of Bet v 1 founds or is part of a superfamily of domains called SRPBCC (START/RHOalphaC/PITP/Bet v1/CoxG/CalC) that include the StAR-related lipid-transfer (START) domain.
Function
The biological function of Bet v 1 is still under investigations. Bet v 1 harbors a large hydrophobic pocket and is able to bind a large spectra of ligands in it like hormones and siderophores like flavenols. It belongs to the pathogenesis-related (PR) proteins, which are usually expressed upon infections and stressful conditions, implicating a role in host defense. In vitro, Bet v 1 has been shown to be immune-suppressive, when it pocket was filled by a ligand, but was able to mount a Th2-response important in allergy development, when the pocket remained empty.
