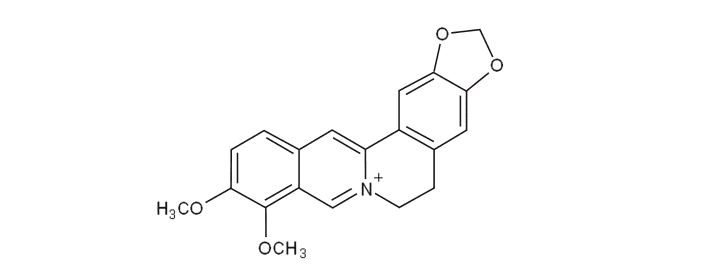
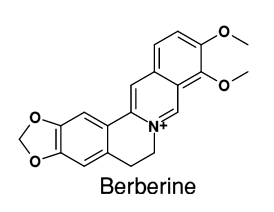
Formula C20H18NO4+ Appearance yellow solid | Molar mass 336.3612 g/mol | |
 | ||
Berberine a powerful natural compound
Berberine is a quaternary ammonium salt from the protoberberine group of benzylisoquinoline alkaloids. It is found in such plants as Berberis (e.g. Berberis vulgaris (barberry), Berberis aristata (tree turmeric)), Mahonia aquifolium (Oregon grape), Hydrastis canadensis (goldenseal), Xanthorhiza simplicissima (yellowroot), Phellodendron amurense (Amur cork tree), Coptis chinensis (Chinese goldthread), Tinospora cordifolia, Argemone mexicana (prickly poppy), and Eschscholzia californica (Californian poppy). Berberine is usually found in the roots, rhizomes, stems, and bark.
Contents
Due to berberine's strong yellow color, Berberis species were used to dye wool, leather, and wood. Wool is still dyed with berberine today in northern India. Under ultraviolet light, berberine shows a strong yellow fluorescence, so it is used in histology for staining heparin in mast cells. As a natural dye, berberine has a colour index of 75160.
Folk medicine
Berberine was supposedly used in China as a folk medicine by Shennong around 3000 BC. This first recorded use of Berberine is described in the ancient Chinese medical book The Divine Farmer's Herb-Root Classic.
Research
Berberine is under investigation to determine whether it may have applications as a drug in treating arrhythmia, diabetes, hyperlipidemia, and cancer. It exerts class III antiarrhythmic action. The bioavailability of berberine is low.
Some research has been undertaken into possible use against MRSA infection.

Berberine is considered antibiotic. When applied in vitro and in combination with methoxyhydnocarpin, an inhibitor of multidrug resistance pumps, berberine inhibits growth of Staphylococcus aureus and Microcystis aeruginosa, a toxic cyanobacterium.
Biosynthesis
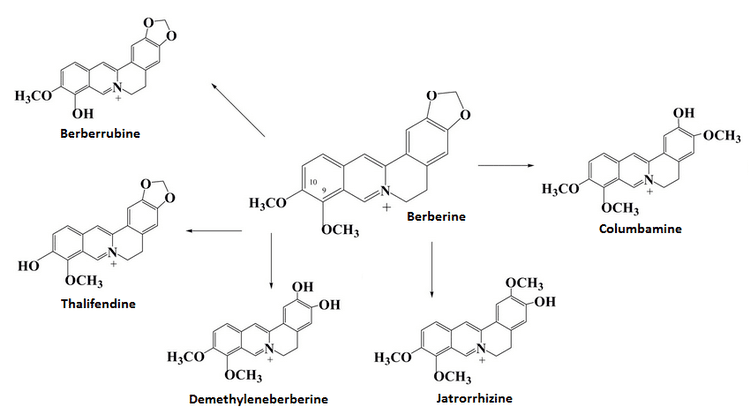
The alkaloid berberine has a tetracyclic skeleton derived from a benzyltetrahydroisoquinoline system with the incorporation of an extra carbon atom provided by S-adenosyl methionine (SAM) via an N-methyl group. Formation of the berberine bridge is readily rationalized as an oxidative process in which the N-methyl group is oxidized to an iminium ion, and a cyclization to the aromatic ring occurs by virtue of the phenolic group.
Reticuline is known as the immediate precursor of protoberberine alkaloids in plants. Berberine is an alkaloid derived from tyrosine. L-DOPA and 4-hydroxypyruvic acid both come from L-tyrosine. Although two tyrosine molecules are used in the biosynthetic pathway, only the phenylethylamine fragment of the tetrahydroisoquinoline ring system is formed via DOPA, the remaining carbon atoms come from tyrosine via 4-hydroxyphenylacetaldehyde. L-DOPA loses carbon dioxide to form dopamine 1. Likewise, 4-hydroxypyruvic acid also loses carbon dioxide to form 4-hydroxyphenylacetaldehyde 2. Dopamine 1 then reacts with 4-hydroxy-phenylacetaldehyde 2 to form (S)-norcolaurine 3 in a reaction similar to the Mannich reaction. After oxidation and methylation by SAM, (S)-reticuline 4 is formed. (S)-reticuline serves as a pivotal intermediate to other alkaloids. Oxidation of the tertiary amine then occurs and an iminium ion is formed 5. In a Mannich-like reaction the ortho position to the phenol is nucleophilic, and electrons are pushed to form 6. Product 6 then undergoes keto-enol tautomerism to form (S)-scoulerine, which is then methylated by SAM to form (S)-tetrahydrocolumbamine 7. Product 7 is then oxidized to form the methylenedioxy ring from the ortho-methoxyphenol, via an O2-, NADPH- and cytochrome P-450-dependent enzyme, giving (S)-canadine 8. (S)-canadine is then oxidized to give the quaternary isoquinolinium system of berberine. This happens in two separate oxidation steps, both requiring molecular oxygen, with H2O2 and H2O produced in the successive processes.
