Formula C13H10O Molar mass 182.217 g/mol Density 1.11 g/cm³ | Melting point 48.5 °C Boiling point 305.4 °C Appearance White solid | |
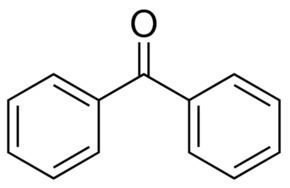 | ||
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.
Contents
- Benzophenone carbonyl compounds class 12 chemistry subject notes lectures cbse iitjee neet
- Uses
- Synthesis
- Organic chemistry
- Benzophenone radical anion
- Commercially significant derivatives
- Pharmacological activity
- References
Benzophenone carbonyl compounds class 12 chemistry subject notes lectures cbse iitjee neet
Uses
Benzophenone can be used as a photo initiator in UV-curing applications such as inks, imaging, and clear coatings in the printing industry. Benzophenone prevents ultraviolet (UV) light from damaging scents and colors in products such as perfumes and soaps.
Benzophenone can also be added to plastic packaging as a UV blocker to prevent photo-degradation of the packaging polymers or its contents. Its use allows manufacturers to package the product in clear glass or plastic (such as a PETE water bottle). Without it, opaque or dark packaging would be required.
In biological applications, benzophenones have been used extensively as photophysical probes to identify and map peptide–protein interactions.
Synthesis
Benzophenone is produced by the copper-catalyzed oxidation of diphenylmethane with air.
A laboratory route involves the reaction of benzene with carbon tetrachloride followed by hydrolysis of the resulting diphenyldichloromethane. It can also be prepared by Friedel-Crafts acylation of benzene with benzoyl chloride in the presence of a Lewis acid (e.g. aluminium chloride) catalyst.
Another route of synthesis is through a palladium(II)/oxometalate catalyst. This converts an alcohol to a ketone with two groups on each side.
Another, less well-known reaction to produce benzophenone is the pyrolysis of anhydrous calcium benzoate.
Organic chemistry
Benzophenone is a common photosensitizer in photochemistry. It crosses from the S1 state into the triplet state with nearly 100% yield. The resulting diradical will abstract a hydrogen atom from a suitable hydrogen donor to form a ketyl radical.
Benzophenone radical anion
Alkali metals reduce benzophenone to the deeply blue colored radical anion, diphenylketyl:
M + Ph2CO → M+Ph2CO•−Generally sodium is used as the alkali metal. Although inferior in terms of safety and effectiveness relative to molecular sieves, this ketyl is used in the purification of organic solvents, particularly ethers, because it reacts with water and oxygen to give non-volatile products. The ketyl is soluble in the organic solvent being dried, so it accelerates the reaction of the sodium with water and oxygen. In comparison, sodium is insoluble, and its heterogeneous reaction is much slower. When excess alkali metal is present a second reduction may occur, resulting in a color transformation from deep blue to purple:
M + M+Ph2CO•− → (M+)2(Ph2CO)2−Commercially significant derivatives
Substituted benzophenones such as oxybenzone and dioxybenzone are used in some sunscreens. The use of benzophenone-derivatives which structurally resemble a strong photosensitizer has been strongly criticized (see sunscreen controversy). Its use in sunscreen has been known to cause coral bleaching via algae blooms, as it washes into the ocean from human use.
Michler's ketone has dimethylamino substituents at each para position.
The high-strength polymer PEEK is prepared from derivatives of benzophenone.
Pharmacological activity
Benzophenone derivatives are known to be pharmacologically active. From a molecular chemistry point of view interaction of benzophenone with B-DNA has been demonstrated experimentally. The interaction with DNA and the successive photo-induced energy transfer is at the base of the benzophenone activity as a DNA photosensitizers and may explain part of its therapeutic potentialities.
In 2014, benzophenones were named Contact Allergen of the Year by the American Contact Dermatitis Society.
