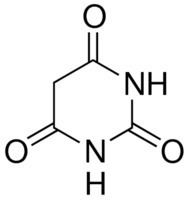
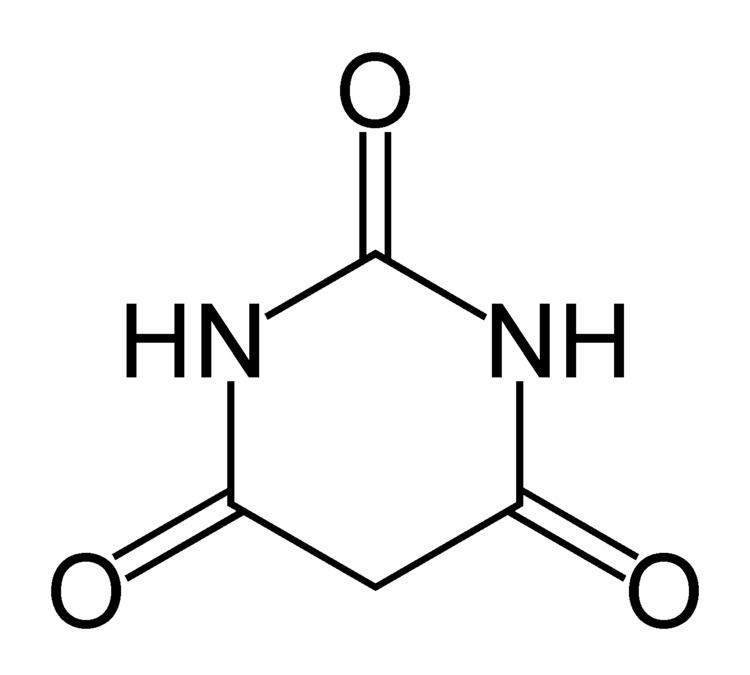
Formula C4H4N2O3 Molar mass 128.09 g/mol Boiling point 260 °C | Melting point 245 °C Appearance White crystals | |
 | ||
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active.
Contents
It remains unclear why the German chemist Adolf Baeyer chose to name the compound that he discovered "barbituric acid". In his textbook Organic Chemistry, the American organic chemist Louis Frederick Fieser (1899–1977) initially speculated that the name stemmed from the German word Schlüsselbart (literally, the beard (Bart ; Latin: barba) of a key (Schlüssel) ; that is, the bit of a key), because Baeyer had regarded barbituric acid as central (or "key") to understanding uric acid and its derivatives. However, Fieser subsequently decided that Baeyer had named the compound after a young lady whom he'd met and who was called "Barbara" ; hence the name "barbituric acid" was a combination of the name "Barbara" and "uric acid". Other sources claim that Baeyer named the compound after Saint Barbara, either because he discovered it on the feast day of St. Barbara (December 4th) or because he sometimes lunched with artillery officers and St. Barbara is their patron saint.
Synthesis
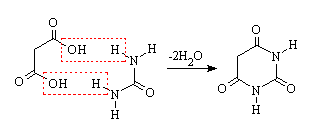
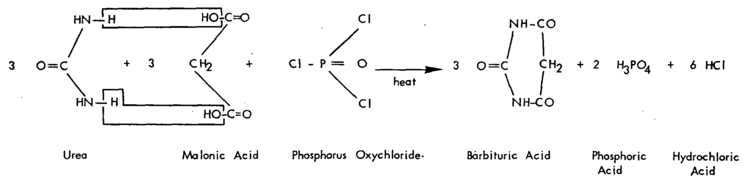
Barbituric acid was first prepared and named in 1863 by the German chemist Adolf von Baeyer, by reducing what Baeyer called Alloxanbromid (alloxan dibromide ; now: 5,5-dibromobarbituric acid) with hydrocyanic acid, and later by reducing dibromobarbituric acid with a combination of sodium amalgam and hydrogen iodide. In 1879, the French chemist Édouard Grimaux synthesized barbituric acid from malonic acid, urea, and phosphorus oxychloride (POCl3). Malonic acid has since been replaced by diethyl malonate, because using the ester avoids the problem of having to deal with the acidity of the carboxylic acid and its unreactive carboxylate.
Properties
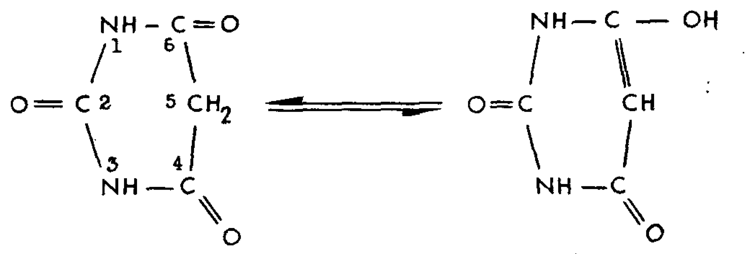
The α-carbon has a reactive hydrogen atom and is quite acidic (pKa = 4.01) even for a diketone species (cf. dimedone with pKa 5.23 and acetylacetone with pKa 8.95) because of the additional aromatic stabilization of the carbanion.
Uses
Using the Knoevenagel condensation reaction, barbituric acid can form a large variety of barbiturate drugs that behave as central nervous system depressants. As of 2007, more than 2550 barbiturates and related compounds have been synthesised, with 50 to 55 in clinical use around the world at present. The first to be used in medicine was barbital (Veronal) starting in 1903, and the second, phenobarbital was first marketed in 1912.
Barbituric acid is one of four ingredients used to make riboflavin (vitamin B2).
Health and safety
Overdose of barbituric acid can cause respiratory problems and death.