Type ? Legal status Investigational ChemSpider none | CAS Number 1537032-82-8 UNII KXG2PJ551I | |
 | ||
Avelumab for treatment of metastatic merkel cell carcinoma
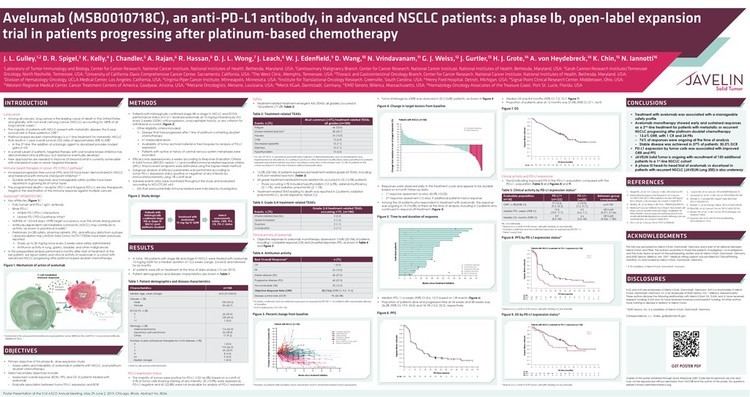
Avelumab (MSB0010718C) is a fully human monoclonal PD-L1 antibody of isotype IgG1, currently in development by Merck KGaA, Darmstadt, Germany & Pfizer for use in immunotherapy, especially for treatment of NSCLC .
Contents
- Avelumab for treatment of metastatic merkel cell carcinoma
- Avelumab in patients with relapsed or recurrent ovarian cancer
- Mechanism of action
- Clinical trials
- References
Avelumab in patients with relapsed or recurrent ovarian cancer
Mechanism of action
Avelumab binds to the PD ligand 1 and therefore inhibits binding to its receptor programmed cell death 1 (PD-1). Formation of a PD-1/PD-L1 receptor/ligand complex leads to inhibition of CD8+ T cells, and therefore inhibition of an immune reaction. Immunotherapy aims at ceasing this immune blockage by blocking those receptor ligand pairs. In the case of avelumab, the formation of PD-1/PDL1 ligand pairs is blocked and CD8+ T cell immune response should be increased. PD-1 itself has also been a target for immunotherapy. Therefore, avelumab belongs to the group of Immune checkpoint blockade cancer therapies.
Clinical trials
As of May 2015, according to Merck KGaA, Darmstadt, Germany & Pfizer, avelumab has been in Phase I clinical trials for bladder cancer, gastric cancer, head and neck cancer, mesothelioma, NSCLC, ovarian cancer and renal cancer. For Merkel-cell carcinoma, Phase II has been reached and for NSCLC there is also a study already in Phase III.
