Species Human Entrez 3906 | Human Mouse Ensembl ENSG00000167531 | |
 | ||
Aliases LALBA, entrez:3906, LYZG, lactalbumin alpha External IDs OMIM: 149750 MGI: 96742 HomoloGene: 1720 GeneCards: LALBA | ||
Lactalbumin, alpha-, also known as LALBA, is a protein that in humans is encoded by the LALBA gene.
Contents
Function
α-Lactalbumin is a protein present in the milk of almost all mammalian species. In primates, alpha-lactalbumin expression is upregulated in response to the hormone prolactin and increases the production of lactose.
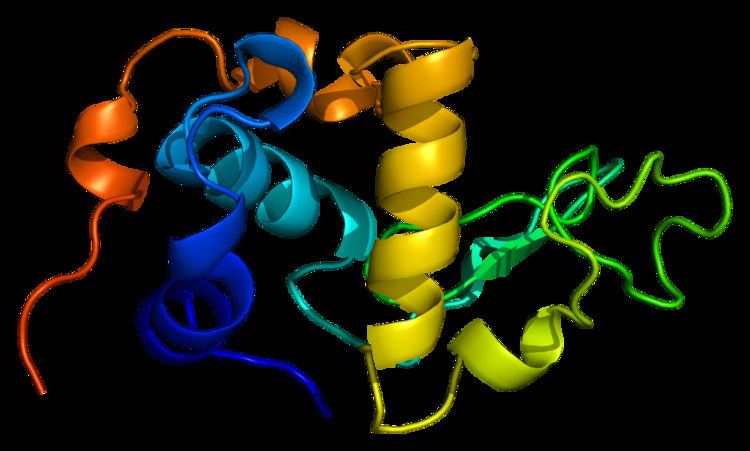
α-Lactalbumin forms the regulatory subunit of the lactose synthase (LS) heterodimer and β-1,4-galactosyltransferase (beta4Gal-T1) forms the catalytic component. Together, these proteins enable LS to produce lactose by transferring galactose moieties to glucose. As a multimer, alpha-lactalbumin strongly binds calcium and zinc ions and may possess bactericidal or antitumor activity. A folding variant of human alpha-lactalbumin that may form in acidic environments such as the stomach, called HAMLET, probably induces apoptosis in tumor and immature cells. The corresponding folding dynamics of alpha-lactalbumin is thus highly unusual.
When formed into a complex with Gal-T1, a galactosyltransferase, α-lactalbumin, enhances the enzyme's affinity for glucose by about 1000 times, and inhibits the ability to polymerise multiple galactose units. This gives rise to a pathway for forming lactose by converting Gal-TI to Lactose synthase.
Physical properties
The structure of alpha-lactalbumin is well known and is composed of 123 amino acids and 4 disulfide bridges. The molecular weight is 14178 Da, and the isoelectric point is between 4.2 and 4.5. One of the main structural differences with beta-lactoglobulin is that it does not have any free thiol group that can serve as the starting-point for a covalent aggregation reaction. As a result, pure α-lactalbumin will not form gels upon denaturation and acidification.
Evolution
The sequence comparison of α-lactalbumin shows a strong similarity to that of lysozymes, specifically the Ca2+-binding c-lysozyme. So the expected evolutionary history is that gene duplication of the c-lysozyme was followed by mutation. This gene predates the last common ancestor of mammals and birds, which probably puts its origin at about 300 Ma.
