EC number 3.2.1.1 IntEnz IntEnz view ExPASy NiceZyme view | CAS number 9000-90-2 BRENDA BRENDA entry KEGG KEGG entry | |
 | ||
α-Amylase is a protein enzyme EC 3.2.1.1 that hydrolyses alpha bonds of large, alpha-linked polysaccharides, such as starch and glycogen, yielding glucose and maltose. It is the major form of amylase found in humans and other mammals. It is also present in seeds containing starch as a food reserve, and is secreted by many fungi.
Contents
In human physiology
Although found in many tissues, amylase is most prominent in pancreatic juice and saliva, each of which has its own isoform of human α-amylase. They behave differently on isoelectric focusing, and can also be separated in testing by using specific monoclonal antibodies. In humans, all amylase isoforms link to chromosome 1p21 (see AMY1A).
Salivary amylase (ptyalin)
Amylase is found in saliva and breaks starch into maltose and dextrin. This form of amylase is also called "ptyalin" /ˈtaɪəlɪn/ It will break large, insoluble starch molecules into soluble starches (amylodextrin, erythrodextrin, and achrodextrin) producing successively smaller starches and ultimately maltose. Ptyalin acts on linear α(1,4) glycosidic linkages, but compound hydrolysis requires an enzyme that acts on branched products. Salivary amylase is inactivated in the stomach by gastric acid. In gastric juice adjusted to pH 3.3, ptyalin was totally inactivated in 20 minutes at 37 °C. In contrast, 50% of amylase activity remained after 150 minutes of exposure to gastric juice at pH 4.3. Both starch, the substrate for ptyalin, and the product (short chains of glucose) are able to partially protect it against inactivation by gastric acid. Ptyalin added to buffer at pH 3.0 underwent complete inactivation in 120 minutes; however, addition of starch at a 0.1% level resulted in 10% of the activity remaining, and similar addition of starch to a 1.0% level resulted in about 40% of the activity remaining at 120 minutes.
Optimum conditions for ptyalin
Optimum pH - 7.0 Human body temperature Presence of certain anions and activators:Genetic variation in human salivary amylase
The salivary amylase gene has undergone duplication during evolution, and DNA hybridization studies indicate many individuals have multiple tandem repeats of the gene. The number of gene copies correlates with the levels of salivary amylase, as measured by protein blot assays using antibodies to human amylase. Gene copy number is associated with apparent evolutionary exposure to high-starch diets. For example, a Japanese individual had 14 copies of the amylase gene (one allele with 10 copies, and a second allele with four copies). The Japanese diet has traditionally contained large amounts of rice starch. In contrast, a Biaka individual carried six copies (three copies on each allele). The Biaka are rainforest hunter-gatherers who have traditionally consumed a low-starch diet. Perry and colleagues speculated the increased copy number of the salivary amylase gene may have enhanced survival coincident to a shift to a starchy diet during human evolution.
Pancreatic amylase
Pancreatic α-amylase randomly cleaves the α(1-4) glycosidic linkages of amylose to yield dextrin, maltose, or maltotriose. It adopts a double displacement mechanism with retention of anomeric configuration.
In pathology
The test for amylase is easier to perform than that for lipase, making it the primary test used to detect and monitor pancreatitis. Medical laboratories will usually measure either pancreatic amylase or total amylase. If only pancreatic amylase is measured, an increase will not be noted with mumps or other salivary gland trauma.
However, because of the small amount present, timing is critical when sampling blood for this measurement. Blood should be taken soon after a bout of pancreatitis pain, otherwise it is excreted rapidly by the kidneys.
Salivary α-amylase has been used as a biomarker for stress that does not require a blood draw.
Interpretation
Increased plasma levels in humans are found in:
Total amylase readings of over 10 times the upper limit of normal (ULN) are suggestive of pancreatitis. Five to 10 times the ULN may indicate ileus or duodenal disease or renal failure, and lower elevations are commonly found in salivary gland disease.
Genes
In grain
α-Amylase activity in grain is measured by, for instance, the Hagberg-Perten Falling Number, a test to assess sprout damages, or the Phadebas method.
Industrial use
α-Amylase is used in ethanol production to break starches in grains into fermentable sugars.
The first step in the production of high-fructose corn syrup is the treatment of cornstarch with α-amylase, producing shorter chains of sugars oligosaccharides.
An α-amylase called "Termamyl", sourced from Bacillus licheniformis, is also used in some detergents, especially dishwashing and starch-removing detergents.
See amylase for more uses of the amylase family in general.
Buffer inhibition
The tris molecule is reported to inhibit a number of bacterial α-amylases, so they should not be used in tris buffer.
Determination
Several methods are available for determination of α-amylase activity, and different industries tend to rely on different methods. The starch iodine test, a development of the iodine test, is based on colour change, as α-amylase degrades starch and is commonly used in many applications. A similar but industrially produced test is the Phadebas amylase test, which is used as a qualitative and quantitative test within many industries, such as detergents, various flour, grain, and malt foods, and forensic biology.
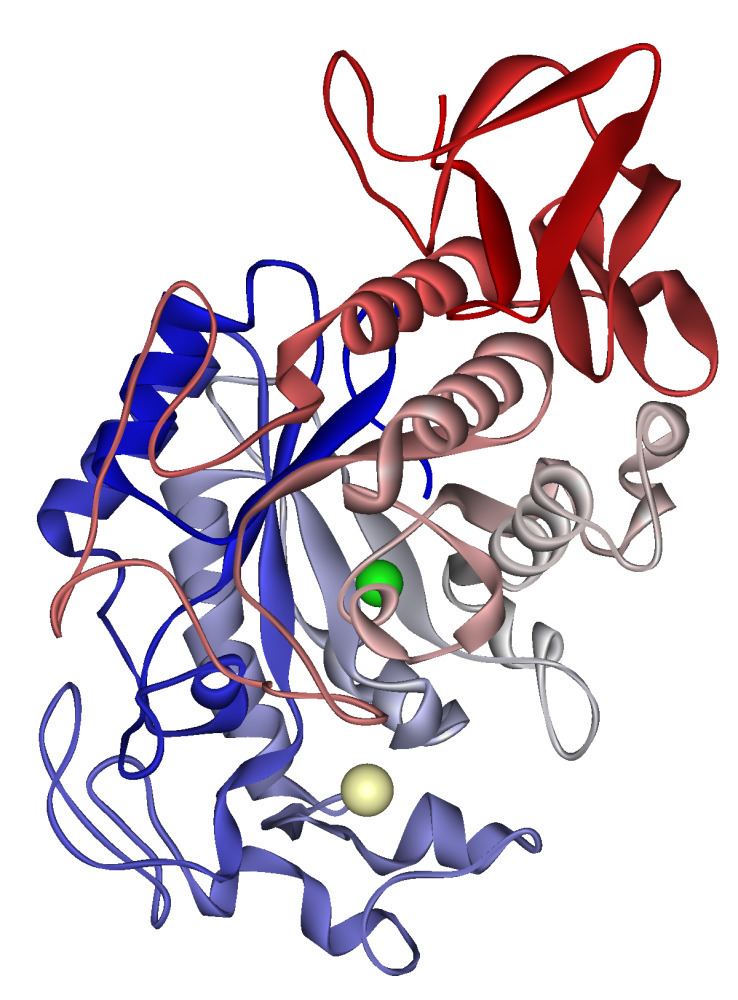
Domain architecture
α-Amylases contain a number of distinct protein domains. The catalytic domain has a structure consisting of an eight-stranded alpha/beta barrel that contains the active site, interrupted by a ~70-amino acid calcium-binding domain protruding between beta strand 3 and alpha helix 3, and a carboxyl-terminal Greek key beta-barrel domain. Several alpha-amylases contain a beta-sheet domain, usually at the C terminus. This domain is organised as a five-stranded antiparallel beta-sheet. Several alpha-amylases contain an all-beta domain, usually at the C terminus.
