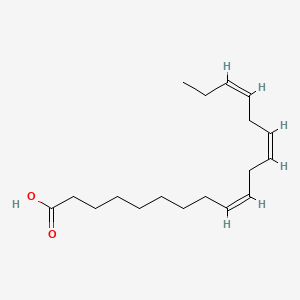
Formula C18H30O2 Density 914 kg/m³ | Molar mass 278.43 g/mol Classification Omega-3 fatty acid | |
 | ||
Alpha linolenic acid inflammation
α-Linolenic acid (ALA) is an n−3 fatty acid. It is one of two essential fatty acids (the other being linoleic acid), so called because they are necessary for health and cannot be produced within the human body. They must be acquired through diet. ALA is an omega-3 fatty acid found in seeds (chia, flaxseed, hemp, see also table below), nuts (notably walnuts), and many common vegetable oils. In terms of its structure, it is named all-cis-9,12,15-octadecatrienoic acid. In physiological literature, it is listed by its lipid number, 18:3, and (n−3); its isomer GLA is 18:3 (n−6).
Contents
- Alpha linolenic acid inflammation
- History
- Potential role in nutrition and health
- Stability and hydrogenation
- Cardiovascular
- References

α-Linolenic acid is a carboxylic acid with an 18-carbon chain and three cis double bonds. The first double bond is located at the third carbon from the methyl end of the fatty acid chain, known as the n end. Thus, α-linolenic acid is a polyunsaturated n−3 (omega-3) fatty acid. It is an isomer of gamma-linolenic acid (GLA), a polyunsaturated n−6 (omega-6) fatty acid.

History

α-Linolenic acid was first isolated by Rollett as cited in J. W. McCutcheon's synthesis in 1942, and referred to in Green and Hilditch's 1930s survey. It was first artificially synthesized in 1995 from C6 homologating agents. A Wittig reaction of the phosphonium salt of [(Z-Z)-nona-3,6-dien-1-yl]triphenylphosphonium bromide with methyl 9-oxononanoate, followed by saponification, completed the synthesis.
Potential role in nutrition and health

Although the best source of ALA is seeds, most seeds and seed oils are much richer in an n−6 fatty acid, linoleic acid. Exceptions include flaxseed (must be ground for proper nutrient absorption) and chia seeds. Linoleic acid is the other essential fatty acid, but it, and the other n−6 fatty acids, compete with n−3s for positions in cell membranes and have very different effects on human health. There is a complex set of essential fatty acid interactions.

α-Linolenic acid can only be obtained by humans through their diets because the absence of the required 12- and 15-desaturase enzymes makes de novo synthesis from stearic acid impossible. Eicosapentaenoic acid (EPA; 20:5, n−3) and docosahexaenoic acid (DHA; 22:6, n−3) are readily available from fish and algae oil and play a vital role in many metabolic processes. These can also be synthesized by humans from dietary α-linolenic acid, but with an efficiency of only a few percent. Because the efficacy of n−3 long-chain polyunsaturated fatty acid (LC-PUFA) synthesis decreases down the cascade of α-linolenic acid conversion, DHA synthesis from α-linolenic acid is even more restricted than that of EPA. Conversion of ALA to DHA is higher in women than in men.
Multiple studies have shown a relationship between α-linolenic acid and an increased risk of prostate cancer. This risk was found to be irrespective of source of origin (e.g., meat, vegetable oil). However, a large 2006 study found no association between total α-linolenic acid intake and overall risk of prostate cancer; and a 2009 meta-analysis found evidence of publication bias in earlier studies, and concluded that if ALA contributes to increased prostate cancer risk, the increase in risk is quite small.
Stability and hydrogenation
α-Linolenic acid is relatively more susceptible to oxidation and will become rancid more quickly than many other oils. Oxidative instability of α-linolenic acid is one reason why producers choose to partially hydrogenate oils containing α-linolenic acid, such as soybean oil. Soybeans are the largest source of edible oils in the U.S., and 40% of soy oil production is partially hydrogenated.

However, when partially hydrogenated, part of the unsaturated fatty acids become unhealthy trans fats. Consumers are increasingly avoiding products that contain trans fats, and governments have begun to ban trans fats in food products. These regulations and market pressures have spurred the development of low-α-linolenic acid soybeans. These new soybean varieties yield a more stable oil that doesn't require hydrogenation for many applications, thus providing trans fat-free products, such as frying oil.
Several consortia are bringing low-α-linolenic acid soy to market. DuPont's effort involves silencing the FAD2 gene that codes for Δ6-desaturase, giving a soy oil with very low levels of both α-linolenic acid and linoleic acid. Monsanto Company has introduced to the market Vistive, their brand of low α-linolenic acid soybeans, which is less controversial than GMO offerings, as it was created via conventional breeding techniques.
Cardiovascular
There is some evidence ALA consumption might have a slight preventative effect against cardiovascular diseases.