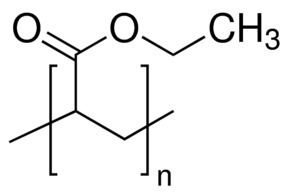
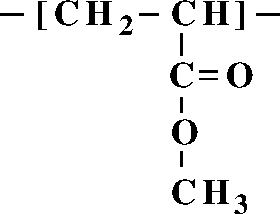Formula C H2=CHCOO− | ||
 | ||
2012 contact allergen of the year acrylates
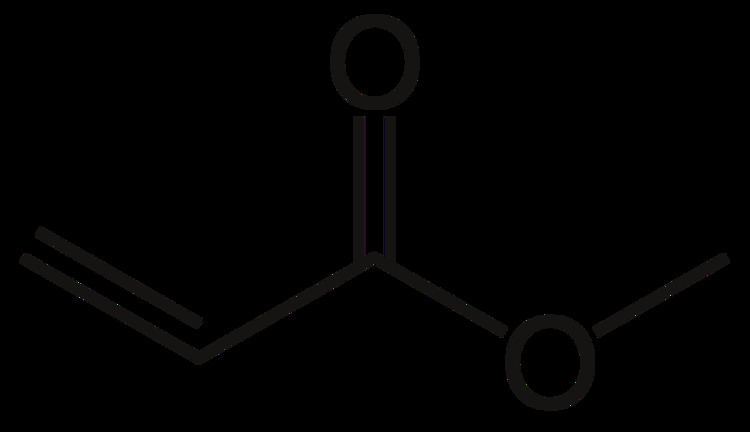
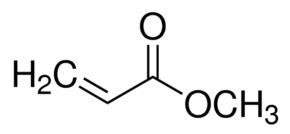
Acrylates are the salts, esters, and conjugate bases of acrylic acid and its derivatives. They are also known as propenoates (since acrylic acid is also known as 2-propenoic acid). The acrylate ion has the molecular formula CH2=CHCOO−. Acrylates contain vinyl groups, that is, two carbon atoms double bonded to each other, directly attached to the carbonyl carbon.
Contents
- 2012 contact allergen of the year acrylates
- Acrylate polymer
- Use
- Production
- Appearance in nature
- References
Acrylate polymer
Use

Acrylates and methacrylates (the salts and esters of methacrylic acid) are common monomers in polymer plastics, forming the acrylate polymers. Acrylates easily form polymers because the double bonds are very reactive.
Recently, organic−inorganic hybrid nanobuilding blocks of acrylate-functionalized polyhedral oligomeric silsesquioxanes (T8, T10, and T12) were easily prepared and separated in their pure forms, which are promising candidate monomers to prepare any hybrid polymer nanocomposites.
Production
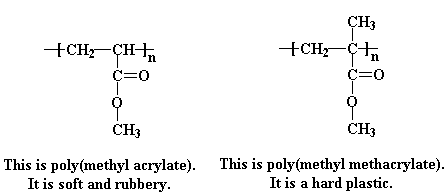
Acrylates are industrially prepared by reacting acrylic acid with the corresponding alcohol in presence of a catalyst. The reaction with lower alcohols (methanol, ethanol) takes place at 100-120 °C with acidic heterogeneous catalysts (cation exchanger). The reaction of higher alcohols (n-butanol, 2-ethylhexanol) is catalysed with sulfuric acid in homogeneous phase. Acrylates of even higher alcohols are obtainable by transesterification of lower esters catalysed by titanium alcoholates or organic tin compounds (e. g. dibutyltin dilaurate).
Appearance in nature
Acrylate has been suggested to be used by marine phytoplankton as a poisonous defense against predators such as protozoa. When attacked, DMSP lyase breaks down dimethylsulfoniopropionate (DMSP) into dimethylsulfide (DMS) and acrylate.