Appearance White powder Melting point 107 °C | Formula C13H9N Boiling point 346 °C | |
 | ||
Similar Acridine carboxamide, Acridine orange, Acridine yellow | ||
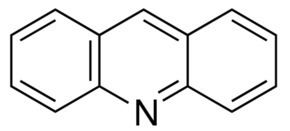
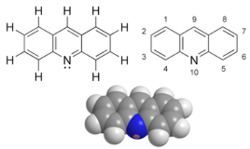
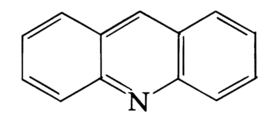
Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.
Contents
Isolation and syntheses
Carl Gräbe and Heinrich Caro first isolated acridine in 1870 from coal tar. Acridine is separated from coal tar by extracting with dilute sulfuric acid. Addition of potassium dichromate to this solution precipitates acridine bichromate. The bichromate is decomposed using ammonia.
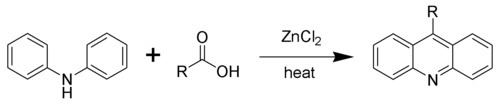
Acridine and its derivatives can be prepared by many synthetic processes. In the Bernthsen acridine synthesis, diphenylamine is condensed with carboxylic acids in the presence of zinc chloride. When formic acid is the carboxylic acid, the reaction yields the parent acridine. With the higher larger carboxylic acids, the derivatives substituted at the meso carbon atom are generated.
Other older methods for the organic synthesis of acridines include condensing diphenylamine with chloroform in the presence of aluminium chloride, by passing the vapours of orthoaminodiphenylmethane over heated litharge, by heating salicylaldehyde with aniline and zinc chloride or by distilling acridone (9-position a carbonyl group) over zinc dust. Another classic method for the synthesis of acridones is the Lehmstedt-Tanasescu reaction.
Reactions

Acridine displays the reactions expected of an unsaturated N-heterocycle. It undergoes N-alkylation with alkyl iodides to form alkyl acridinium iodides, which are readily transformed by the action of alkaline potassium ferricyanide to N-alkyl acridones.
Basicity
Acridine and its homologues are weakly basic. Acridine is a photobase which have a ground state pKa of 5.1, which is similar to that of pyridine, and have an excited state pKa of 10.6. It also shares properties with quinoline.
Reduction and oxidation
Acridines can be reduced to the 9,10-dihydroacridines, sometimes called leuco acridines. Reaction with potassium cyanide gives the 9-cyano-9,10-dehydro derivative. On oxidation with potassium permanganate, it yields acridinic acid (C9H5N(CO2H)2) otherwise known as quinoline-1,2-dicarboxylic acid. Acridine is easily oxidized by peroxymonosulfuric acid to the acridine amine oxide. The carbon 9-position of acridine is activated for addition reactions.
Applications
Several dyes and drugs feature the acridine skeleton. Many acridines, such as proflavine, also have antiseptic properties. Acridine and related derivatives (such as amsacrine) bind to DNA and RNA due to their abilities to intercalate. Acridine orange (3,6-dimethylaminoacridine) is a nucleic acid-selective metachromatic stain useful for cell cycle determination.
Dyes
At one time acridine dyes were commercially significant, but they are now uncommon because they are not lightfast. Acridine dyes are prepared by condensation of 1,3-diaminobenzene derivatives. Illustrative is the reaction of 2,4-diaminotoluene with acetaldehyde:
9-Phenylacridine is the parent base of chrysaniline or 3,6-diamino-9-phenylacridine, which is the chief constituent of the dyestuff phosphine (not to be confused with phosphine gas), a by-product in the manufacture of rosaniline. Chrysaniline forms red-coloured salts, which dye silk and wool in a fine yellow; and the solutions of the salts are characterized by their fine yellowish-green fluorescence. Chrysaniline was synthesized by O. Fischer and G. Koerner by condensing ortho-nitrobenzaldehyde with aniline, the resulting ortho-nitro-para-diamino-triphenylmethane being reduced to the corresponding orthoamino compound, which on oxidation yields chrysaniline. Benzoflavin, an isomer of chrysaniline, is also a dye-stuff, and has been prepared by K. Oehler from meta-phenylenediamine and benzaldehyde. These substances condense to form tetra-aminotriphenylmethane, which, on heating with acids, loses ammonia and yields 3,6-diamino-9,10-dihydrophenylacridine, from which benzoflavin is obtained by oxidation. It is a yellow powder, soluble in hot water.
Safety
Acridine is a skin irritant. Its LD50 (rats, oral) is 2000 mg/kg and 500 mg/kg (mice, oral).
