Formula C2H2O2 | ||
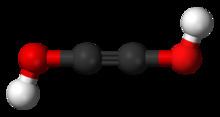 | ||
Acetylenediol, or ethynediol, is a chemical substance with formula HO-C≡C-OH. It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal H(C=O)2H is well known.
Contents
Detection
Acetylenediol was first observed in the gas-phase by mass spectrometry. The compound was later obtained by photolysis of squaric acid in a solid argon matrix at 10 K (−263.1 °C).
Alkoxide derivatives
Although the diol has only fleeting existence in concentrated form, salts of the acetylenediolate (ethynediolate) dianion (O-C≡C-O)2− are well known. These organometallic compounds (specifically, alkoxides) are formally derived from ethynediol by loss of two hydrogen ions, but they are not normally generated in that way.
The typical synthesis route for these salts is the reduction of carbon monoxide. Potassium acetylenediolate (K2C2O2) was first obtained by Liebig in 1834, from the reaction of carbon monoxide with metallic potassium; but for a long time the product was assumed to be "potassium carbonyl" (KCO). Over the next 130 years were described the "carbonyls" of sodium (Johannis, 1893), barium (Gunz and Mentrel, 1903), strontium (Roederer, 1906), and lithium, rubidium, and caesium (Pearson, 1933). The reaction was eventually shown to yield a mixture of the potassium acetylenediolate K
2C
2O
2 and potassium benzenehexolate K
6C
6O
6.
The true structure of these salts was clarified only in 1963 by Werner Büchner and E. Weiss.
Acetylenediolates can also be prepared by the rapid reaction of CO and a solution of the corresponding metal in liquid ammonia at low temperature. Potassium acetylenediolate is a pale yellow solid that reacts explosively with air, halogens, halogenated hydrocarbons, alcohols, water, and any substance which possesses an acidic hydrogen.
Coordination complexes
Acetylenediol can form coordination compounds, such as [TaH(HOC≡COH)(dmpe)2Cl]+Cl− where dmpe is bis(dimethylphosphino)ethane.
Acetylenediolate and related anions such as deltate C
3O2−
3 and squarate C
4O2−
4 have been obtained from carbon monoxide under mild conditions by reductive coupling of CO ligands in organouranium complexes.
Other derivatives
Although again not derived from acetylenediol, a variety of structurally related compounds are known. Examples include the diethers diisopropoxyethyne ((CH3)2CH)-O-C≡C-O-(CH(CH3)2) and di-tert-butoxyethyne ((CH3)3C)-O-C≡C-O-(C(CH3)3).
