Formula C8H16O2 Appearance Colorless liquid | Density 903 kg/m³ | |
 | ||
Related compounds | ||
2015 deep research report on global 2 ethylhexanoic acid industry
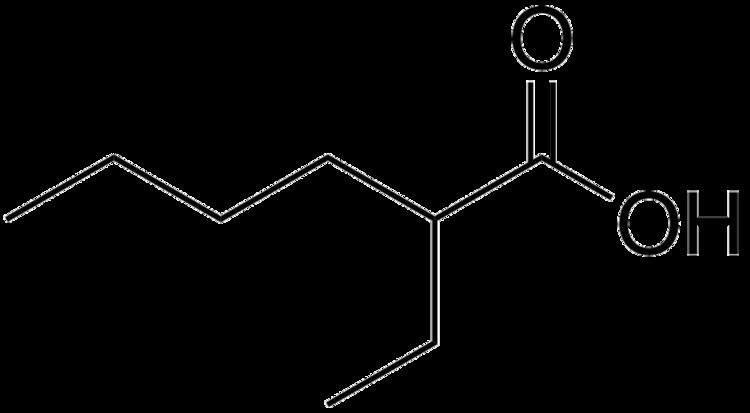
2-Ethylhexanoic acid is the organic compound with the formula CH3(CH2)3CH(C2H5)CO2H. It is a carboxylic acid that is widely used to prepare lipophilic metal derivatives that are soluble in nonpolar organic solvents. 2-Ethylhexanoic acid is a colorless viscous oil. It is supplied as a racemic mixture.
Contents
- 2015 deep research report on global 2 ethylhexanoic acid industry
- Metal ethylhexanoates
- Examples of metal ethylhexanoates
- References
2-Ethylhexanoic acid is produced industrially in two steps from butyraldehyde. Aldol condensation of the aldehyde gives 2-ethylhexanal. Oxidation of the latter gives the carboxylic acid.
Metal ethylhexanoates
These lipophilic metal-containing derivatives are used in many ways as catalysts in polymerizations, oxidation (drying agents), and organic synthesis. The high solubility of these metal complexes is attributable to the long hydrocarbon chain and the presence of a chiral center which leads to mixtures of enantiomeric complexes. These metal complexes, which exist as mixtures of several diastereoisomer, are often described as salts. They are, however, not ionic but charge-neutral coordination complexes. Their structures are akin to the corresponding acetates.
