Formula C3H10N2 | Appearance Colourless liquid | |
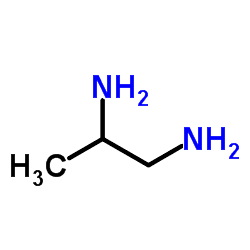 | ||
Related alkanamines Related compounds | ||
1,2-Diaminopropane (1,2-propanediamine) is a diamine commonly used as a bidentate ligand in coordination chemistry. Pn is the simplest chiral diamine. The molecules exists as a colorless liquid at room temperature.
Contents
Preparation
Industrially, this compound is synthesized by the ammonolysis of 1,2-dichloropropane: This preparation allows for the use of waste chloro-organic compounds to form useful amines using inexpensive and readily available ammonia:
CH3CHClCH2Cl + 4 NH3 → CH3CH(NH2)CH2NH2 + 2 NH4ClThe racemic mixture of this chiral compound may be separated into enantiomers by conversion into the diastereomeric tartaric acid ammonium salt. After purification of the diastereomer, the diamine can be regenerated by treatment of the ammonium salt with sodium hydroxide. Alternate reagents for chiral resolution include N-p-toluenesulfonylaspartic acid, N-benzenesulfonylaspartic acid, or N-benzoylglutamic acid.
N,N-Disalicylidene-1,2-propanediamine
1,2-Diaminopropane can be converted to N,N′-disalicylidene-1,2-propanediamine, a useful salen-type ligand that is abbreviated salpn. The synthesis is achieved by a condensation reaction of the diamine with salicylaldehyde:
2C6H4(OH)CHO + CH3CH(NH2)CH2NH2 → [C6H4(OH)CH]2CH3CHNCH2N + 2H2OSalpn is used as a fuel additive as a metal deactivator in motor oils. Trace metals degrade the fuels by catalyzing oxidation processes that lead to gums and solids. Metal deactivators like salpn form stable complexes with the metals, suppressing their catalytic activity. While salpn forms stable chelate complexes with many metals including copper, iron, chromium, and nickel, it is the coordination with copper that makes it a popular choice as a fuel additive. Copper has the highest catalytic activity in fuel, and salpn forms a highly stable square-planar complex with the metal. This complex is especially stable because salpn is a tetra-dentate ligand with a -2 charge (after deprotonation of the two phenolic groups).
The use of salpn is preferred over the use of ethylenediamine (producing salen), possibly because the propane derivatives has higher solubility.
