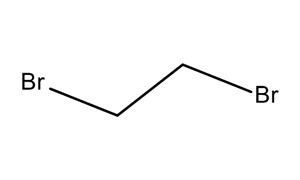
Abbreviations EDB Formula C2H4Br2 Boiling point 131.4 °C Classification Heavy liquid | Appearance Colorless liquid Density 2.17 g/cm³ Molar mass 187.86 g/mol | |
Related alkanes | ||
Free rotation in 1 2 dibromoethane mp4
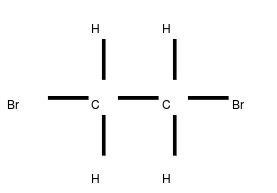
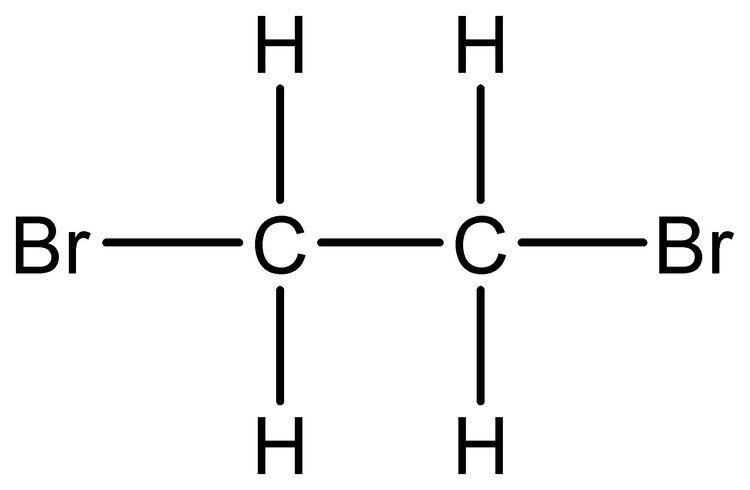
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is the organobromine compound with the chemical formula (CH2Br)2. Although trace amounts occur naturally in the ocean, where it is formed probably by algae and kelp, it is mainly synthetic. It is a colorless liquid with a sweet odor, detectable at 10 ppm, is a widely used and sometimes-controversial fumigant.
Contents
Preparation and use
It is produced by the reaction of ethylene with bromine, in a classic halogen addition reaction:
CH2=CH2 + Br2 → BrCH2CH2Br
Historically, 1,2-dibromoethane was used as an anti-knock additive in leaded fuels. It reacts with lead residues to generate volatile lead bromides, thereby preventing fouling of the engine.
Pesticide
It has been used as a pesticide in soil and on various crops. The applications were initiated after the forced retirement of 1,2-dibromo-3-chloropropane (DBCP). Most of these uses have been stopped in the U.S. It continues to be used as a fumigant for treatment of logs for termites and beetles, for control of moths in beehives.
Reagent
Ethylene bromide has wider applications in the preparation of other organic compounds. It is used to make vinyl bromide, a precursor to some fire retardants.
In the laboratory, 1,2-dibromoethane is used in organic synthesis as a source of bromine, e.g., to brominate carbanions and to activate magnesium for certain Grignard reagents. In the latter process, the 1,2-dibromoethane is converted to ethylene and magnesium bromide, exposing a freshly etched portion of magnesium to the substrate.
Health effects
The effects on people of breathing high levels are not known, but animal studies with short-term exposures to high levels caused depression and collapse, indicating effects on the brain. Changes in the brain and behavior were also seen in young rats whose male parents had breathed 1,2-dibromoethane, and birth defects were observed in the young of animals that were exposed while pregnant. 1,2-Dibromoethane is not known to cause birth defects in humans. Swallowing has caused death at 40 mL doses
It is a known carcinogen, with pre-1977 exposure levels ranking it as the most carcinogenic substance on the HERP Index.
