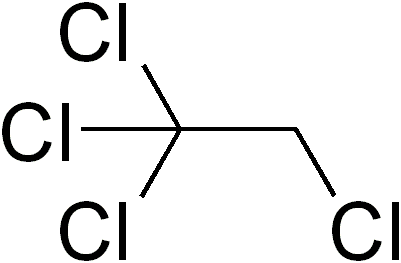
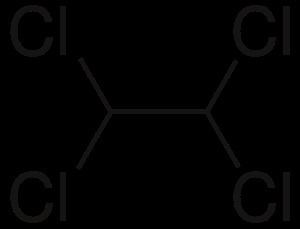
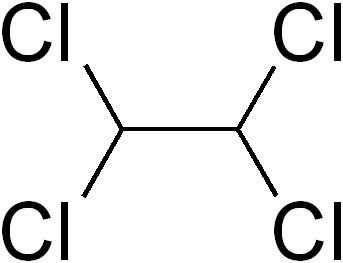
Formula C2H2Cl4 Density 1.59 g/cm³ | Molar mass 167.848 g/mol | |
 | ||
Appearance Colorless to pale yellow liquid | ||
1,1,2,2-Tetrachloroethane is a chlorinated derivative of ethane. It has the highest solvent power of any chlorinated hydrocarbon. As a refrigerant, it is used under the name R-130.
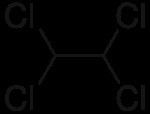
It was once widely used as a solvent and as an intermediate in the industrial production of trichloroethylene, tetrachloroethylene, and 1,2-dichloroethylene. However, 1,1,2,2-tetrachloroethane is no longer used much in the United States due to concerns about its toxicity.

Chronic inhalation exposure in humans results in jaundice and an enlarged liver, headaches, tremors, dizziness, numbness, and drowsiness. The U.S. Environmental Protection Agency has classified it as a Group C possible human carcinogen.
For occupational exposure limits, the Occupational Safety and Health Administration has set a permissible exposure limit for dermal exposures at 5 ppm over an eight-hour time-weighted average. The National Institute for Occupational Safety and Health has a more protective recommended exposure limit for dermal exposures at 1 ppm over an eight-hour time-weighted average.