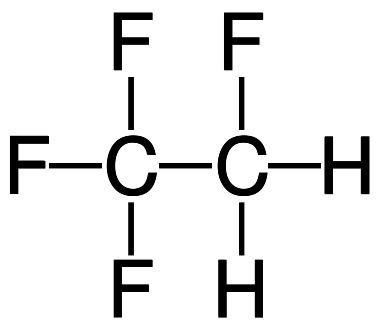
Appearance Colorless gas IUPAC ID 1,1,1,2-Tetrafluoroethane Melting point -103.3 °C Molar mass 102.03 g/mol | Formula CH2FCF3 Boiling point -26.3 °C Density 4.25 kg/m³ Soluble in Water | |
 | ||
Related compounds Thermodynamicdata Phase behavioursolid–liquid–gas | ||
1 1 1 2 tetrafluoroethane r 134 a surface tension
1,1,1,2-tetrafluoroethane, R-134a, Forane 134a, Genetron 134a, Florasol 134a, Suva 134a or HFC-134a, also known as norflurane (INN), is a haloalkane refrigerant with thermodynamic properties similar to R-12 (dichlorodifluoromethane) but with insignificant ozone depletion potential and a somewhat lower global warming potential (1300, compared to R-12's GWP of 2400). It has the formula CH2FCF3 and a boiling point of −26.3 °C (−15.34 °F) at atmospheric pressure. R-134a cylinders are colored light blue. Attempts at phasing out its use as a refrigerant with substances that have lower global warming potentials, such as HFO-1234yf are underway.
Contents
- 1 1 1 2 tetrafluoroethane r 134 a surface tension
- Refrigerant 134 a 1 1 1 2 tetrafluoroethane latent heat and temperature
- Uses
- Climate change considerations
- United States
- History
- Safety
- Use in inhalers
- References

Refrigerant 134 a 1 1 1 2 tetrafluoroethane latent heat and temperature
Uses
1,1,1,2-Tetrafluoroethane is a non-flammable gas used primarily as a "high-temperature" refrigerant for domestic refrigeration and automobile air conditioners. These devices began using 1,1,1,2-tetrafluoroethane in the early 1990s as a replacement for the more environmentally harmful R-12 and retrofit kits are available to convert units that were originally R-12-equipped. Other uses include plastic foam blowing, as a cleaning solvent, a propellant for the delivery of pharmaceuticals (e.g. bronchodilators), wine cork removers, gas dusters and in air driers for removing the moisture from compressed air. 1,1,1,2-Tetrafluoroethane has also been used to cool computers in some overclocking attempts. It is also commonly used as a propellant for airsoft airguns. The gas is often mixed with a silicon-based lubricant.
1,1,1,2-tetrafluoroethane is also being considered as an organic solvent suitable for extraction of flavor and fragrance compounds, as a possible alternative to other organic solvents and supercritical carbon dioxide. It can also be used as a solvent in organic chemistry, both in liquid and supercritical fluid. It is used in the resistive plate chamber particle detectors in the Large Hadron Collider. It is also used for other types of particle detectors, e.g. some cryogenic particle detectors. It can be used as an alternative to sulfur hexafluoride in magnesium smelting as a shielding gas.

1,1,1,2-tetrafluoroethane is also being considered as an alternative to sulfur hexafluoride as a dielectric gas. Its arc-quenching properties are poor, but its dielectric properties are fairly good.
Climate change considerations
Recently, 1,1,1,2-tetrafluoroethane has been subject to use restrictions due to its contribution to climate change. It has a global warming potential of 1300.
United States
The Society of Automotive Engineers (SAE) has proposed 1,1,1,2-tetrafluoroethane (HFC-134a) to be best replaced by a new fluorochemical refrigerant HFO-1234yf (CF3CF=CH2) in automobile air-conditioning systems. California may also prohibit the sale of canned 1,1,1,2-tetrafluoroethane to individuals to avoid non-professional recharge of air conditioners. A ban had been in place in Wisconsin since October 1994 under ATCP 136 prohibiting sales of container sizes holding less than 15 lbs of 1,1,1,2-tetrafluoroethane, but this restriction applies only when the chemical is intended to be a refrigerant. The ban has been lifted in Wisconsin in 2012. It appears, for example, that it is legal for a person to purchase gas duster containers with any amount of the chemical because in that instance the chemical is neither intended to be a refrigerant nor is HFC-134a included in the § 7671a listing of class I and class II substances.
History
1,1,1,2-Tetrafluoroethane first appeared in the early 1990s as a replacement for dichlorodifluoromethane (R-12), which has ozone depleting properties. 1,1,1,2-Tetrafluoroethane has been atmospherically modeled for its impact on depleting ozone and as a contributor to global warming. Research suggests that over the past 10 years the concentration of 1,1,1,2-tetrafluoroethane has increased significantly in the Earth's atmosphere, with a recent study revealing a doubling in atmospheric concentration between 2001 and 2004. It has insignificant ozone depletion potential (ozone layer), significant global warming potential (100-yr GWP = 1430) and negligible acidification potential (acid rain). Because of its high GWP, 1,1,1,2-tetrafluoroethane has been banned from use in the European Union, starting with cars in 2011 and phasing out completely by 2017.
Safety
Mixtures with air of the gas 1,1,1,2-tetrafluoroethane are not flammable at atmospheric pressure and temperatures up to 100°C (212°F). However, mixtures with high concentrations of air at elevated pressure and/or temperature can be ignited. Contact of 1,1,1,2-tetrafluoroethane with flames or hot surfaces in excess of 250 °C (482 °F) may cause vapor decomposition and the emission of toxic gases including hydrogen fluoride and carbonyl halides. 1,1,1,2-Tetrafluoroethane itself has an LD50 of 1,500 g/m3 in rats, making it relatively non-toxic, apart from the dangers inherent to inhalant abuse. Its gaseous form is denser than air and will displace air in the lungs. This can result in asphyxiation if excessively inhaled. This is what contributes to most deaths by inhalant abuse.
Aerosol cans containing 1,1,1,2-tetrafluoroethane, when inverted, become effective freeze sprays. Under pressure, 1,1,1,2-tetrafluoroethane is compressed into a liquid, which upon vaporization absorbs a significant amount of thermal energy. As a result, it will greatly lower the temperature of any object it contacts as it evaporates. This can result in frostbite when it contacts skin, as well as blindness upon eye contact.
Use in inhalers
1,1,1,2-tetrafluoroethane and HFA227ea are propellants of pressurised metered dose inhalers (pMDI) used by asthmatics.
This chemical is an inhalational anaesthetic studied only in animals in 1967, and toxicology investigations did not acknowledge this fact. All hydrofluorocarbon inhaled anaesthetic agents have smooth muscle relaxing properties, so the propellant gas produced by pMDIs using HFA134a and HFA227ea (which has no anaesthetic activity) will add to beta2-agonist bronchodilating drugs from that inhaler.
The change from inert chlorofluorocarbon freon propellant to HFAs occurred in the late 1990s, and coincided with the fact that "Asthmatic athletes have consistently outperformed healthy athletes in every Olympic games since 2000".
