 | ||
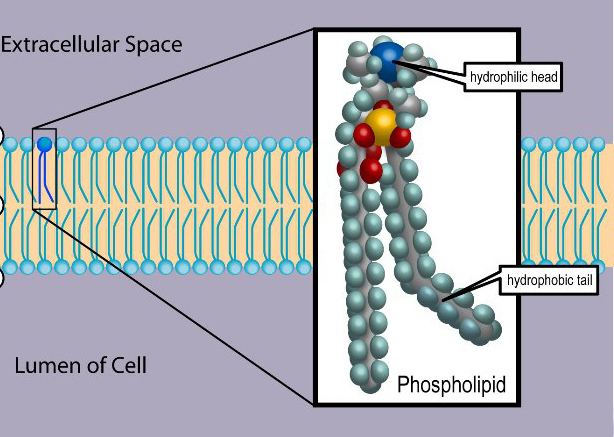
Phospholipids are a class of lipids that are a major component of all cell membranes. They can form lipid bilayers because of their amphiphilic characteristic. The structure of the phospholipid molecule generally consists of two hydrophobic fatty acid "tails" and a hydrophilic "head" consisting of a phosphate group. The two components are joined together by a glycerol molecule. The phosphate groups can be modified with simple organic molecules such as choline.
Contents
- Amphiphilic character
- Diacylglyceride structures
- Phosphosphingolipids
- Applications
- Simulations
- Characterization
- Analysis
- Phospholipid synthesis
- In signal transduction
- Food technology
- Phospholipid derivatives
- References
The first phospholipid identified in 1847 as such in biological tissues was lecithin, or phosphatidylcholine, in the egg yolk of chickens by the French chemist and pharmacist, Theodore Nicolas Gobley. Biological membranes in eukaryotes also contain another class of lipid, sterol, interspersed among the phospholipids and together they provide membrane fluidity and mechanical strength. Purified phospholipids are produced commercially and have found applications in nanotechnology and materials science.
Amphiphilic character
An amphiphile (from the Greek αμφις, amphis: both and φιλíα, philia: love, friendship) is a term describing a chemical compound possessing both hydrophilic (water-loving, polar) and lipophilic (fat-loving) properties. The phospholipid head contains a negatively charged phosphate group and glycerol; it is hydrophilic (attracted to water). The phospholipid tails usually consists of 2 long fatty acid chains; they are hydrophobic and are repelled by water. When placed in water, phospholipids form a variety of structures depending on the specific properties of the phospholipid with tails typically aggregating to minimize interactions with water molecules. These specific properties allow phospholipids to play an important role in the phospholipid bilayer. In biological systems, the phospholipids often occur with other molecules (e.g., proteins, glycolipids, sterols) in a bilayer such as a cell membrane. Lipid bilayers occur when hydrophobic tails line up against one another, forming a membrane of hydrophilic heads on both sides facing the water.
Such movement can be described by the fluid mosaic model, that describes the membrane as a mosaic of lipid molecules that act as a solvent for all the substances and proteins within it, so proteins and lipid molecules are then free to diffuse laterally through the lipid matrix and migrate over the membrane. Sterols contribute to membrane fluidity by hindering the packing together of phospholipids. However, this model has now been superseded, as through the study of lipid polymorphism it is now known that the behaviour of lipids under physiological (and other) conditions is not simple.
Diacylglyceride structures
See: GlycerophospholipidPhosphosphingolipids
See Sphingolipid
Applications
Phospholipids have been widely used to prepare liposomal, ethosomal and other nanoformulations of topical, oral and parenteral drugs for differing reasons like improved bio-availability, reduced toxicity and increased penetration. Liposomes are often composed of phosphatidylcholine-enriched phospholipids and may also contain mixed Phospholipid chains with surfactant properties. The ethosomal formulation of ketoconazole using phospholipids is a promising option for transdermal delivery in fungal infections.
Simulations
Computational simulations of phospholipids are often performed using molecular dynamics with force fields such as GROMOS, CHARMM, or AMBER.
Characterization
Phospholipids are optically highly birefringent, i.e. their refractive index is different along their axis as opposed to perpendicular to it. Measurement of birefringence can be achieved using cross polarisers in a microscope to obtain an image of e.g. vesicle walls or using techniques such as dual polarisation interferometry to quantify lipid order or disruption in supported bilayers.
Analysis
There are no simple methods available for analysis of phospholipids since the close range of polarity between different phospholipid species makes detection difficult. Oil chemists often use spectroscopy to determine total Phosphorus abundance and then calculate approximate mass of phospholipids based on molecular weight of expected fatty acid species. Modern lipid profiling employs more absolute methods of analysis, with nuclear magnetic resonance spectroscopy (NMR), particularly 31P-NMR, while HPLC-ELSD provides relative values.
Phospholipid synthesis
Phospholipid synthesis occurs in the cytosole adjacent to ER membrane that is studded with proteins that act in synthesis (GPAT and LPAAT acyl transferases, phosphatase and choline phosphotransferase) and allocation (flippase and floppase). Eventually a vesicle will bud off from the ER containing phospholipids destined for the cytoplasmic cellular membrane on its exterior leaflet and phospholipids destined for the exoplasmic cellular membrane on its inner leaflet.
In signal transduction
Some types of phospholipid can be split to produce products that function as second messengers in signal transduction. Examples include phosphatidylinositol (4,5)-bisphosphate (PIP2), that can be split by the enzyme Phospholipase C into inositol triphosphate (IP3) and diacylglycerol (DAG), which both carry out the functions of the Gq type of G protein in response to various stimuli and intervene in various processes from long term depression in neurons to leukocyte signal pathways started by chemokine receptors.
Phospholipids also intervene in prostaglandin signal pathways as the raw material used by lipase enzymes to produce the prostaglandin precursors. In plants they serve as the raw material to produce Jasmonic acid, a plant hormone similar in structure to prostaglandins that mediates defensive responses against pathogens.BMB'63.
Food technology
Phospholipids can act as emulsifiers, enabling oils to form a colloid with water. Phospholipids are one of the components of lecithin which is found in egg-yolks, as well as being extracted from soy beans, and is used as a food additive in many products, and can be purchased as a dietary supplement. Lysolecithins are typically used for water-oil emulsions like margarine, due to their higher HLB ratio.
