Symbol RIP InterPro IPR001574 SCOP 1paf | Pfam PF00161 PROSITE PDOC00248 SUPERFAMILY 1paf | |
 | ||
A ribosome-inactivating protein is a protein synthesis inhibitor that acts at the ribosome.
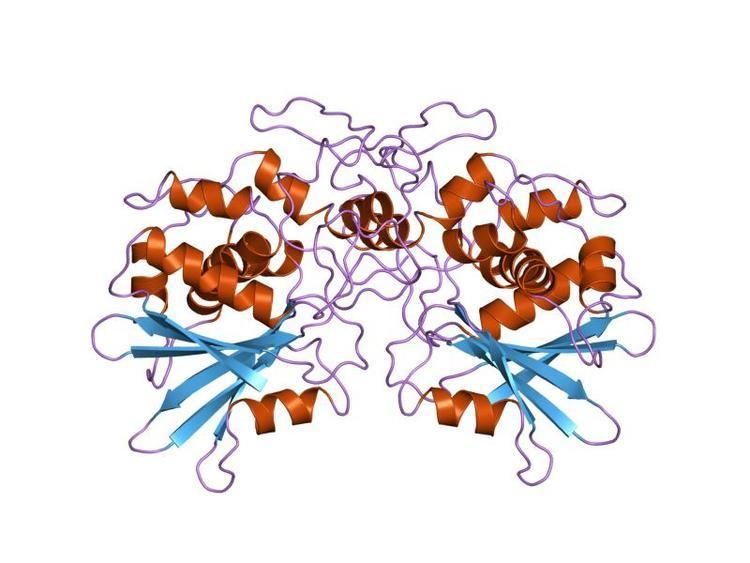
A number of bacterial and plant toxins act by inhibiting protein synthesis in eukaryotic cells. The toxins of the Shiga and ricin family inactivate 60S ribosomal subunits by an N-glycosidic cleavage, which releases a specific adenine base from the sugar-phosphate backbone of 28S rRNA. Members of the family include shiga and shiga-like toxins, and type I (e.g. trichosanthin and luffin) and type II (e.g. ricin, agglutinin and abrin) ribosome inactivating proteins (RIPs). All these toxins are structurally related. RIPs have been of considerable interest because of their potential use, conjugated with monoclonal antibodies, as immunotoxins to treat cancers. Further, trichosanthin has been shown to have potent activity against HIV-1-infected T cells and macrophages. Elucidation of the structure-function relationships of RIPs has therefore become a major research effort. It is now known that RIPs are structurally related. A conserved glutamic residue has been implicated in the catalytic mechanism; this lies near a conserved arginine, which also plays a role in catalysis.
Examples include:
They exist in bacteria and plants.
It should also be noted that only a minority of RIPs are toxic to humans when consumed, and proteins of this family are found in the vast majority of plants used for human consumption, such as Rice, Maize and Barley.
