Density 1.1 g/cm³ | Appearance white solid | |
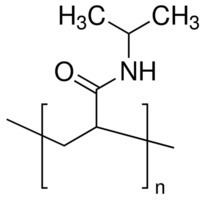 | ||
Poly(N-isopropylacrylamide) (variously abbreviated PNIPA, PNIPAAm, NIPA, PNIPAA or PNIPAm) is a temperature-responsive polymer that was first synthesized in the 1950s. It can be synthesized from N-isopropylacrylamide which is commercially available. It is synthesized via free radical polymerization and is readily functionalized making it useful in a variety of applications.
Contents
- History
- Chemical and Physical Properties
- Synthesis of Heat and pH Sensitive PNIPA
- Synthesis of Chain End Functionalized PNIPA
- Applications
- References
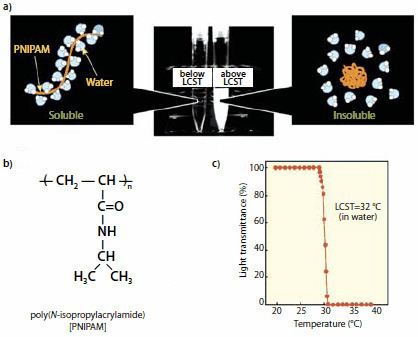
It forms a three-dimensional hydrogel when cross-linked with N,N’-methylene-bis-acrylamide (MBAm) or N,N’-cystamine-bis-acrylamide (CBAm). When heated in water above 32 °C (90 °F), it undergoes a reversible lower critical solution temperature (LCST) phase transition from a swollen hydrated state to a shrunken dehydrated state, losing about 90% of its volume. Since PNIPA expels its liquid contents at a temperature near that of the human body, PNIPA has been investigated by many researchers for possible applications in tissue engineering and controlled drug delivery.
History
The synthesis of poly(N-isoproylacrylamide) first began with the synthesis of acrylamides monomer by Sprecht in 1956. In 1957, Shearer patented the first application for what would be later identified as PNIPA for the use as a rodent repellent. Early work was piqued by theoretical curiosity of the material properties of PNIPA. The first report of PNIPA came in 1968, which elucidated the unique thermal behavior in aqueous solutions. The 1980s marked an explosion in interest in PNIPAs with the realization of potential applications due to its unique thermal behavior in aqueous solutions.
Chemical and Physical Properties
PNIPA is one of the most studied thermosensitive hydrogels. In dilute solution, it undergoes a coil-to-globule transition. PNIPA possesses an inverse solubility upon heating. It changes hydrophilicity and hydrophobicity and abruptly at its LCST. At lower temperatures PNIPA orders itself in solution in order to hydrogen bond with the already arranged water molecules. The water molecules must reorient around the nonpolar regions of PNIPA which results in a decreased entropy. At lower temperatures, such as room temperature, the negative enthalpy term (
causing the PNIPA to absorb water and dissolve in solution. At higher temperatures, the entropy term (
Synthesis of Heat and pH Sensitive PNIPA
Homopolymerization
The process of free radical polymerization of a single type of monomer, in this case, N-isopropylacrylamide, to form the polymer is known as a homopolymerization. The radical initiator azobisisobutyronitrile (AIBN) is commonly used in radical polymerizations.Copolymerization
A free radical polymerization of two different monomer results in a copolymerization. An advantage to a copolymerization includes fine tuning of the LCST.Terpolymerization
A free radical polymerization of three different monomer is known as a terpolymerization. Advantages to a terpolymerization may include enhancing multiple properties of the polymer including thermosensitivity, pH sensitivity or fine tuning of the LCST.Cross-linked Hydrogel
The reaction scheme below is a terpolymerization to form a cross-linked hydrogel. The reactant ammonium persulfate (APS) is used in polymer chemistry as a strong oxidizing agent that is often used along with tetramethylethylenediamine (TMEDA) to catalyze the polymerization when making polyacrylamide gels.Synthesis of Chain-End Functionalized PNIPA
PNIPA can be functionalized using chain transfer agents using a free radical polymerization. The three schemes below demonstrate functionalization using chain transfer agents (CTA), where one end of the polymer is the radical initiator and the other is a functionalized group. Functionalization of the polymer chain-end allows for the polymer to be used in many diverse settings and applications. Advantages to a functionalizing the chain-end may include enhancing multiple properties of the polymer including thermosensitivity, pH sensitivity or fine tuning of the LCST.
(1)
(2)
(3)
Applications
The versatility of PNIPA has led to finding uses in macroscopic gels, microgels, membranes, sensors, biosensors, thin films, tissue engineering, and drug delivery. The tendency of aqueous solutions of PNIPA to increase in viscosity in the presence of hydrophobic molecules has made it excellent for tertiary oil recovery.
Adding additives or copolymerization of PNIPA can lower the lower critical solution temperature to temperatures around human body temperatures, which makes it an excellent candidate for drug delivery applications. The PNIPA can be placed in a solution of bioactive molecules, which allows the bioactive molecules to penetrate the PNIPA. The PNIPA can then be placed in vivo, where there is a rapid release of biomolecules due to the initial gel collapse and an ejection of the biomolecules into the surrounding media, followed by a slow release of biomolecules due to surface pore closure.
PNIPA have also been used in pH-sensitive drug delivery systems. Some examples of these drug delivery systems may include the intestinal delivery of human calcitonin, delivery of insulin, and the delivery of ibuprofen. When radiolabeled PNIPA copolymers with different molecular weights were intravenously injected to rats, it was found that the glomerular filtration threshold of the polymer was around 32 000 g/mol.
PNIPA have been used in gel actuators, which convert external stimuli into mechanical motion. Upon heating above the LCST, the hydrogel goes from hydrophilic to hydrophobic state. This conversion results in an expulsion of water which causes a physical conformational change, creating a mechanical hinge movement.
