Formula C20H30Cl4Ir2 Appearance orange solid | Molar mass 796.71 g/mol | |
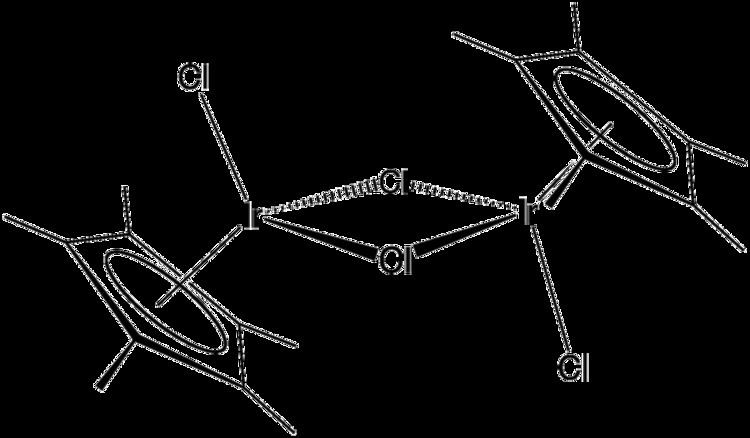 | ||
Pentamethylcyclopentadienyl iridium dichloride is an organometallic compound with the formula [(C5(CH3)5IrCl2)]2, commonly abbreviated [Cp*IrCl2]2 This bright orange air-stable diamagnetic solid is a reagent in organometallic chemistry.
Preparation, structure, reactions
The compound has C2h symmetry. Each metal is pseudo-octahedral. It was first prepared by the reaction of hydrated iridium trichloride with hexamethyl Dewar benzene. More conveniently, the compound is prepared by the reaction of iridium trihydrate and pentamethylcyclopentadiene in hot methanol, from which the product precipitates
2 Cp*H + 2 IrCl3(H2O)3 → [Cp*IrCl2]2 + 2 HCl + 6 H2OThe two Ir-μ-Cl bonds are labile and can be cleaved to give a variety of adducts of the general formula Cp*IrCl2L. Such adducts undergo further substitution to afford cations [Cp*IrClL2]+ and [Cp*IrL3]2+. The chloride ligands can also be replaced by other anions such as carboxylates, nitrite, and azide.
Reduction of [Cp*IrCl2]2 under an atmosphere of CO affords the dicarbonyl Cp*Ir(CO)2], which can be decarbonylated to give the unsaturated derivative [Cp*Ir(CO)]2. Treatment of [Cp*IrCl2]2 with borohydride under an atmosphere of H2 gives the iridium(V) derivative Cp*IrH4.
[Cp*IrCl2]2 is a precursor to catalysts for the asymmetric transfer hydrogenation of ketones.
[Cp*IrCl2]2 can be safely handled under air.
