 | ||
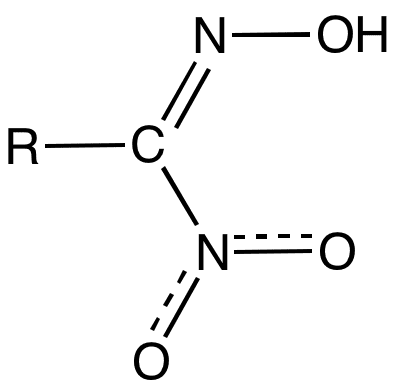
Nitrolic acids are organic compounds with the functional group RC(NO2)=NOH. They are prepared by the reaction of nitroalkanes with base and nitrite sources:
The conversion was first demonstrated by Victor Meyer using nitroethane. The reaction proceeds via the intermediacy of the nitronate anion.
Occurrence
Most nitrolic acids are laboratory curiosities. One exception is the compound HO2C(CH2)4C(NO2)=NOH, which is produced by the oxidation of cyclohexanone with nitric acid. This species decomposes to adipic acid and nitrous oxide:
HO2C(CH2)4C(NO2)=NOH → HO2C(CH2)4CO2H + N2OThis conversion is thought to be the largest anthropogenic route to N2O. Adipic acid is a precursor to many nylon polymers.
References
Nitrolic acid Wikipedia(Text) CC BY-SA
