 | ||
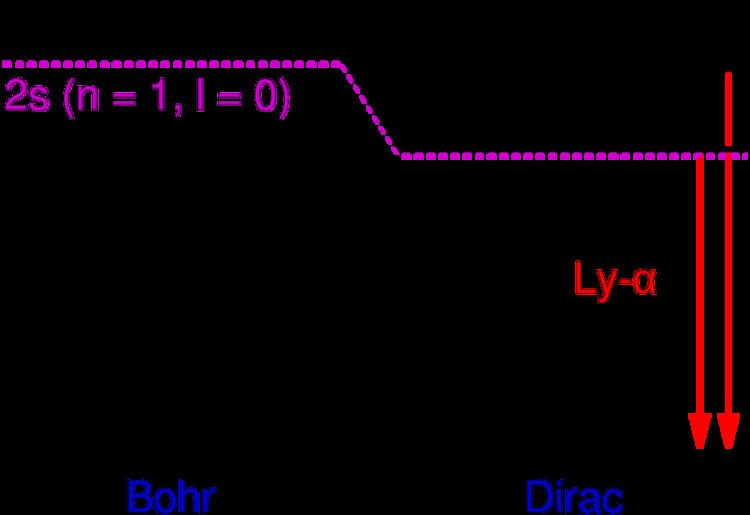
In physics, the Lyman-alpha line, sometimes written as Ly-α line, is a spectral line of hydrogen, or more generally of one-electron ions, in the Lyman series, emitted when the electron falls from the n = 2 orbital to the n = 1 orbital, where n is the principal quantum number. In hydrogen, its wavelength of 1215.67 angstroms (121.567 nm or 6993121567000000000♠1.21567×10−7 m), corresponding to a frequency of 7015247000000000000♠2.47×1015 hertz, places the Lyman-alpha line in the vacuum ultraviolet part of the electromagnetic spectrum. Lyman-alpha astronomy must therefore ordinarily be carried out by satellite-borne instruments, except for extremely distant sources whose redshifts allow the hydrogen line to penetrate the atmosphere.
Because of fine structure perturbations, the Lyman-alpha line splits into a doublet with wavelengths 1215.668 and 1215.674 angstroms. Specifically, because of the electron's spin-orbit interaction, the stationary eigenstates of the perturbed Hamiltonian must be labeled by the total angular momentum j of the electron (spin plus orbital), not just the orbital angular momentum l. In the n = 2 orbital, there are two possible states, j = 1/2 and j = 3/2, resulting in a spectral doublet. The j = 3/2 state is of higher energy (less negative) and so is energetically farther from the n = 1 orbital to which it is transitioning. Thus, the j = 3/2 state is associated with the more energetic (shorter wavelength) spectral line in the doublet.
The less energetic spectral line has been measured at 7015246606141318703♠2466061413187035(10) Hz, or 6993121567312313021♠1215.673123130217(5) Å.
A K-alpha line, or Kα, analogous to the Lyman-alpha line for hydrogen, occurs in the high-energy induced emission spectra of all chemical elements, since it results from the same electron transition as in hydrogen. The equation for the frequency of this line (usually in the X-ray range for heavier elements) uses the same base-frequency as Lyman-alpha, but multiplied by a (Z − 1)2 factor to account for the differing atomic numbers (Z) of heavier elements, as approximated by Moseley's law.
The Lyman-alpha line is most simply described by the {n,m} = {1,2...} solutions to the empirical Rydberg formula for hydrogen's Lyman spectral series. (The Lyman-alpha frequency is produced by multiplying the Rydberg frequency for the atomic mass of hydrogen, RM (see Rydberg constant), by a factor of (1/1)2 − (1/2)2 = 3/4.) Empirically, the Rydberg equation is in turn modeled by the semi-classical Bohr model of the atom.
