Formula LiBO2 Melting point 849 °C | Molar mass 49.751 g/mol Density 2.22 g/cm³ | |
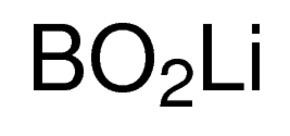 | ||
Appearance white hygroscopic monoclinic crystals | ||
Lithium metaborate (LiBO2) is a chemical compound.
Applications
Lithium metaborate or lithium tetraborate (Li2B4O7), or a mixture of both, can be used in borate fusion sample preparation of various samples for analysis by XRF, AAS, ICP-OES, ICP-AES and ICP-MS.
Simultaneous determination of parts-per-million level Cr, As, Cd and Pb, and major elements in low level contaminated soils using borate fusion and energy dispersive X-ray fluorescence spectrometry with polarized excitation.
Lithium metaborate dissolves acidic oxides such as SiO2 and Fe2O3, where the stoichiometric ratio of oxygen to cation, y/x in MxOy, is greater than unity. Lithium tetraborate dissolves basic oxides such as CaO, MgO and other oxides of the alkali metals and alkaline earth metals, where y/x ≤ 1. Most oxides are best dissolved in a mixture of the two lithium borate salts, for spectrochemical analysis.
