Boiling point 323 °C | ||
 | ||
Hexachlorocyclohexane (HCH) is any of several polyhalogenated organic compounds consisting of a six-carbon ring with one chlorine and one hydrogen attached to each carbon. There are many isomers for this structure, differing by the stereochemistry of the individual chlorine substituents on the cyclohexane. It is sometimes called erroneously called "benzene hexachloride" (BHC). They have been used as models for analyzing the effects of different geometric positions of the large atoms with dipolar bonds on the stability of the cyclohexane conformation. Some isomers are pesticides
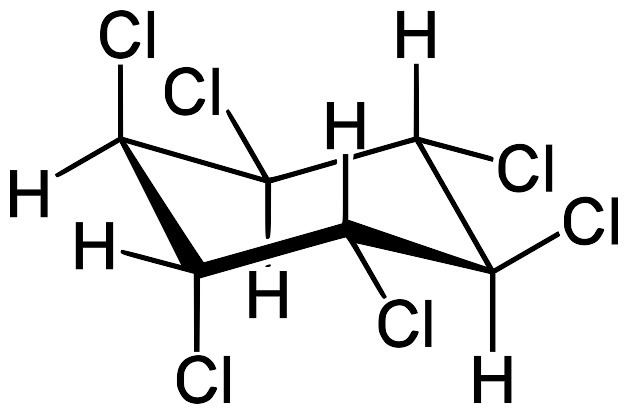
Common forms are:

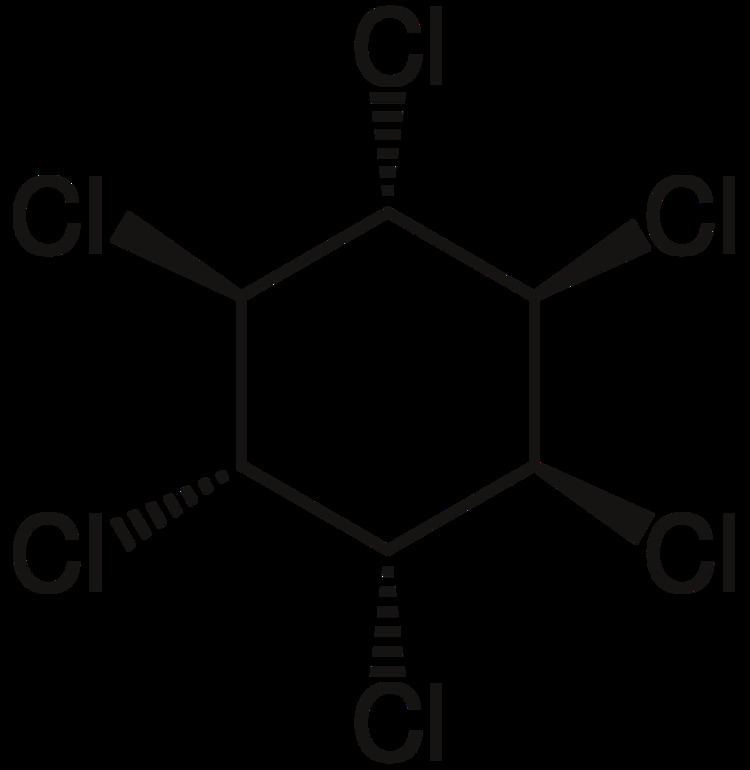
References
Hexachlorocyclohexane Wikipedia(Text) CC BY-SA
