 | ||
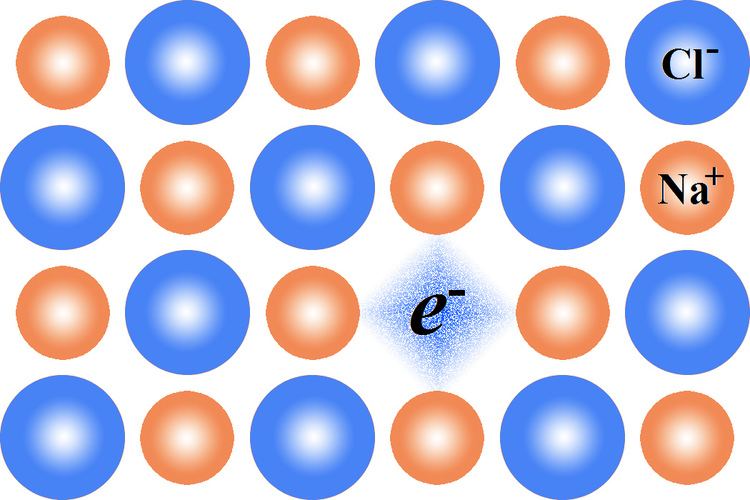
An F-center, Farbe center or color center (from the original German Farbzentrum; Farbe means color, and zentrum center) is a type of crystallographic defect in which an anionic vacancy in a crystal is filled by one or more unpaired electrons. Electrons in such a vacancy tend to absorb light in the visible spectrum such that a material that is usually transparent becomes colored. This is used to identify many compounds, especially zinc oxide (yellow).
Color centers can occur naturally in compounds (particularly metallic oxides) because when heated to high temperature the ions become excited and are displaced from their normal crystallographic positions, leaving behind some electrons in the vacated spaces.
F-centers are often paramagnetic and can then be studied by electron paramagnetic resonance techniques. The greater the number of F-centers, the more intense is the color of the compound. A way of producing F-centers is to heat a crystal in the presence of an atmosphere of the metal that constitutes the material, e.g., NaCl heated in a metallic Na atmosphere.
Na0 → Na+ + e−
Na+ is incorporated at NaCl crystal.
Cl− vacancies are generated, because of the excess of Na+.
These vacancies capture available electrons, e−, to maintain charge neutrality, forming F-centers; that is, the electrons released in this process diffuse to occupy the vacant sites. Ionizing radiation can also produce F-centers.
An H-center (a halogen interstitial) is in a sense the opposite to an F-center, and hence the two can combine and clear the lattice of a defect. This process can be photoinduced, e.g., using a laser.
This is the reason that some crystals like lithium chloride, potassium chloride, and zinc oxide become pink, lilac and yellow, respectively on heating them.
