Symbol Lys-AminoMut_A InterPro IPR015130 PDB RCSB PDB; PDBe; PDBj | Pfam PF09043 Pfam structures PDBsum structure summary | |
 | ||
In enzymology, a D-lysine 5,6-aminomutase (EC 5.4.3.4) is an enzyme that catalyzes the chemical reaction
Contents
- Nomenclature
- Background
- Reactions
- Subunits
- Cofactors
- Activity
- Catalytic cycle
- Structure based catalysis
- History
- References
Hence, this enzyme has one substrate, D-lysine, and one product, 2,5-diaminohexanoate.
This enzyme participates in lysine degradation. It employs one cofactor, cobamide.
Nomenclature
This enzyme belongs to the family of isomerases, specifically those intramolecular transferases transferring amino groups. The systematic name of this enzyme class is D-2,6-diaminohexanoate 5,6-aminomutase. Other names in common use include D-α-lysine mutase and adenosylcobalamin-dependent D-lysine 5,6-aminomutase, which can be abbreviated as 5,6-LAM.
Background
In the early 1950s it was discovered that under anaerobic conditions lysine undergoes degradation to equimolar amounts of acetate and butyrate by an amino-acid-fermenting bacteria Clostridium sticklandii.
Later by implementing isotopic studies two possible pathways were discovered. In pathway A both acetate and butyrate are generated from C2-C3 cleavage of D-lysine. Unlike pathway A, pathway B involves C5-C4 degradation producing the same products.
D-lysine 5,6-aminomutase (5,6-LAM) is responsible for the first conversion in pathway B to turn D-α-lysine into 2,5-diaminohexanoate. Unlike other members of the family of aminomutases (like 2,3-LAM) who are peculiar to a single substrate, 5,6-LAM can reversibly catalyze both the reaction of D-lysine to 2,5-diaminohexanoic acid and the reaction of L-β-lysinene to 3,5-diaminohexanoic acid.
Reactions
5,6-LAM is capable of reversibly catalyzing the migration of amino group from ε-carbon to δ-carbon in both D-lysine and L-β-lysine, and migrating hydrogen atoms from δ-carbon to ε-carbon at the same time.
Subunits
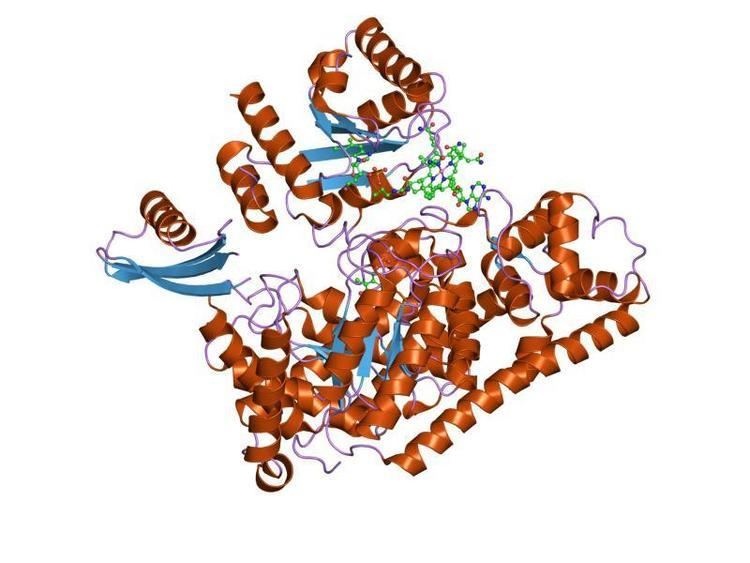
5,6-LAM is an α2β2 tetramer. The structure of the alpha subunit is predominantly a PLP-binding TIM barrel domain, with several additional alpha-helices and beta-strands at the N and C termini. These helices and strands form an intertwined accessory clamp structure that wraps around the sides of the TIM barrel and extends up toward the Ado ligand of the Cbl cofactor, which is the beta subunit providing most of the interactions observed between the protein and the Ado ligand of the Cbl, suggesting that its role is mainly in stabilising AdoCbl in the precatalytic resting state. The β subunit binds AdoCbl while the PLP directly binds to α subunit. But PLP also has a direct binding with Lys144 of β part to form an internal aldimine. PLP and AdoCbl is about 24Å away from each other.
Cofactors
- 5,6-LAM is pyridoxal-5'-phosphate (PLP) dependent and PLP binds to substrate with an external aldimine linkage. PLP is also important to stabilize the radical intermediate by captodative stabilization and spin delocalization.
- Catalysis begins with 5'-deoxyadenosyl radical (Ado•) and 5'-deoxyadenosylcobalamin (AdoCbl) is an essential cofactor as a hydrogen carrier.
- ATP, a mercaptan and a divalent metal ion (usually Mg2+) are required to achieve the highest catalytic effect.
Activity
5,6-LAM shows best catalytic activity in 20mM Tris•HCl with pH around 9.0-9.2.
Catalytic cycle
The catalytic cycle starts with Ado• (5'-deoxyadenosyl radical derived from adenosylcobalamine) subtracting a hydrogen atom from PLP-D-lysine adduct (substrate-related precursor SH) to generate a substrate-related radical (S•), with radical being at carbon 5 of the Lysine residue. The latter undergoes an internal cyclization/addition to the imine nitrogen producing aziridinecarbinyl radical (I•) — a more thermodynamically stable intermediate with a radical being on a benzylic position. Rearrangement of I• produces a product-related radical (P•), which then participates in a final step of hydrogen transfer from AdoH to afford the PLP-product complex (PH).
Structure-based catalysis
A more detailed understanding of the catalytic mechanism can be derived from the X-ray structure.
First, an evident conformational change is observed after the substrate is added to the system. With a substrate-free enzyme the distance between AdoCbl and PLP is about 24 Å and PLP participates in multiple non-covalent interactions with the enzyme with 5,6-LAM presenting an “open” state. For example, in wild type 5,6-LAM, the phenol ring of Tyr263α is oriented in a slipped geometry with pyridine ring of PLP, generating π-π stacking interaction, which is capable of modulating the electron distribution of the high-energetic radical intermediate.
Thus, the first step of the catalytic cycle would be the enzyme accepting the substrate by forming an external aldimine with PLP replacing the PLP-Lys144β internal aldimine. With the cleavage of the internal aldimine, the β unit is able to swing towards to the top of the α unit and block the empty site. Therefore, generation of Ado• radical leads to a change in the structure of the active domain bringing AdoCbl and PLP-substrate complex closer to each other, locking the enzyme in a “closed” state. The closed state exists until the radical transfer occurs when the product is released and AdoCbl is reformed and at the same time, the closed state is transited to open state again to wait for next substrate.
What’s also interesting is the locking mechanism to prevent radical reaction without the presence of substrate discovered by Catherine Drennan’s group. Lys144 β residue is located at a short G-rich loop highly conserved across all 5,6-LAMs, which blocks the AdoCbl from the reaction site. Based on the X-ray structure analysis, when open structure is applied, the axes of the TIM barrel and Rossmann domains are in different directions. And with the addition of the substrate, the subunits rearrange to turn the axes into each other to facilitate the catalysis.
History
Early insights into the mechanism of the catalytic reaction mainly focused on isotopic methods. Both pathways of lysine degradation and the role of 5,6-LAM were discovered in early works by Stadtman’s group during 1950s-1960s. In 1971 having a tritiated α-lysine, 2,5-diaminohexanoate and coenzyme in hand Colin Morley and T. Stadtman discovered the role of 5'-deoxyadenosylcobalamin (AdoCbl) as a source for hydrogen migration. Recently, a lot of progress was done towards detecting the intermediates of the reaction, especially towards I•. Based on quantum-mechanical calculations, it was proposed that with 5-fluorolysine as a substitute for D-lysine the 5-FS• species can be captured and analyzed. Similar approach was applied towards PLP modification, when it was modified to 4’-cyanoPLP or PLP-NO and the radical intermediate I• analogue is hypothesized to be easily detected to support the proposed mechanism. Other simulations can also provide some insights into the catalytic reaction.
