Formula C15H21CrO6 Density 1.34 g/cm³ Appearance deep maroon | Melting point 216 °C Boiling point 340 °C | |
 | ||
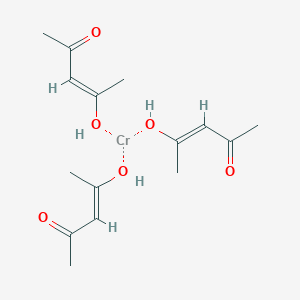
Chromium(III) acetylacetonate is the coordination compound with the formula Cr(C5H7O2)3, sometimes designated as Cr(acac)3. This purplish coordination complex is used in NMR spectroscopy as a relaxation agent because of its solubility in nonpolar organic solvents and its paramagnetism.

Synthesis and structure
The compound is prepared by the reaction of chromium(III) oxide with acetylacetone (Hacac):
Cr2O3 + 6 Hacac → 2 Cr(acac)3 + 3 H2O
The complex has idealized D3 symmetry. The Cr-O distances are 1.93 Å. Like other Cr(III) compounds, it has the d3 configuration, having a quartet ground state. Although it is relatively inert toward substitution, the complex undergoes bromination at the 3-positions of the chelate rings.
References
Chromium(III) acetylacetonate Wikipedia(Text) CC BY-SA
