 | ||
Carbene radicals are a recently proposed class of organometallic carbene. The carbene radical state is formed from reaction between an open-shell transition metal porphyrin and organic ligand. In 2003, experimental work on Cobalt(II) cyclopropanation catalysts suggested the hypothesis that a carbene radical intermediate state was being generated in situ. Theoretical calculations validated this hypothesis and explained the bonding mechanism underlying the stability of the carbene radical. To date, carbene radicals have been synthesized only as long-lived reactive intermediates. The chemical bond present in carbene radicals is surprising in that it possesses aspects of both Fischer and Schrock carbenes.
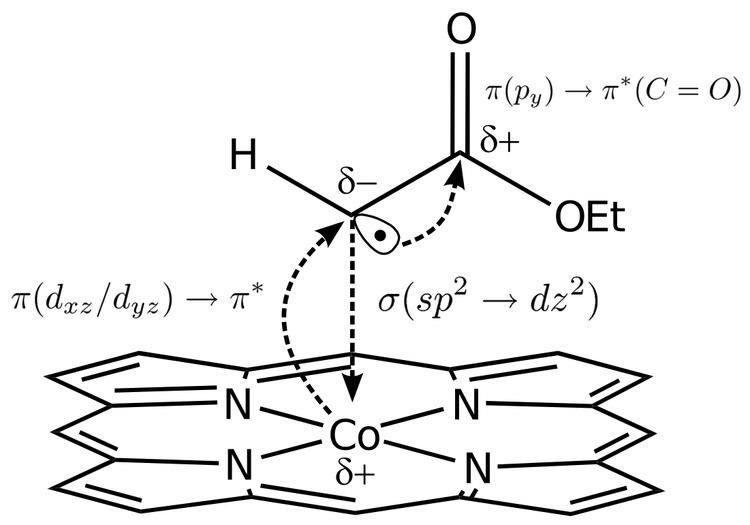
Bonding mechanism of the carbene radical
The mechanism of formation of a carbene radical, like the Fischer mechanism, involves electron donation to a σ molecular orbital of which a dz2 orbital on the metal is a participant. But unlike the Fischer bond, the transition metal dz2 orbital is initially singly occupied with some resulting spin pairing with a donated electron, giving the bond partial Schrock-like character.
In order for the σ bond to be stabilized (typically with a bond order slightly less than 1), a back-bonding action from the π molecular orbital to the anti-bonding π* molecular orbital is necessary and the porphyrin ring serves as an electron π-symmetry "buffer" to ensure this interaction is obtained.
The back-donation to the π* orbital would result in unfavorable excess electron density on the carbene carbon but the presence of adjacent functional groups (carbonyl or sulfonyl groups have the desired electronegativity) relieve this electron build-up and yield the final radical electron, which occupies a single p atomic orbital state on the carbon.
