Molar mass 383.798 g/mol | Appearance Purple | |
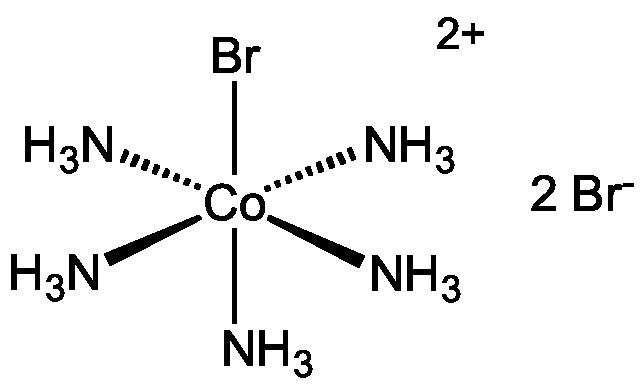 | ||
Bromopentaamminecobalt(III) bromide is the dibromide salt of the cobalt coordination compound with the formula [Co(NH3)5Br]2+ It is a purple, water-soluble solid. The analogous Chloropentaamminecobalt(III) chloride is also well known.
Synthesis and reactions
The title compound is prepared by oxidation of a solution of cobalt(II) salts in aqueous ammonia.
2 CoBr2 + 8 NH3 + 2 NH4Br + H2O2 → 2 [Co(NH3)5Br]Br2 + 2 H2OIt was first reported in the 1870s, before the structure or even formulae were understood for such complexes. This early work showed that only two thirds of the bromide groups were exchangeable with other anions such as nitrate and dithionate.
The complex undergoes aquation, meaning that bromide is displaced by water:
[Co(NH3)5Br]Br2 + H2O → [Co(NH3)5(H2O)]Br3This process is catalyzed by platinum.
References
Bromopentaamminecobalt(III) bromide Wikipedia(Text) CC BY-SA
