Formula C16H24Ni Melting point 60 °C | Molar mass 275.06 g/mol Appearance Yellow solid | |
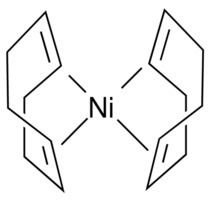 | ||
Bis(cyclooctadiene)nickel(0) is the organometallic compound with the formula Ni(C8H12)2. This air-sensitive yellow solid is a common source of Ni(0) in chemical synthesis.
Ni(cod)2, as it is commonly abbreviated, is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. The complex is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin:
1/3 [Ni3(acac)6] + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4Ni(cod)2 is moderately soluble in benzene and THF. The cod ligands are easily displaced by phosphines, phosphites, and isocyanides.
References
Bis(cyclooctadiene)nickel(0) Wikipedia(Text) CC BY-SA
