Formula C3H6S3 Appearance Colourless solid | Density 1.64 g/cm³ | |
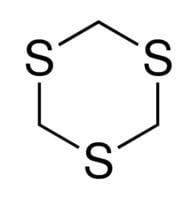 | ||
1,3,5-Trithiane is the chemical compound with the formula (CH2S)3. This heterocycle is the cyclic trimer of the otherwise unstable species thioformaldehyde. It consists of a six-membered ring with alternating methylene bridges and thioether groups. It is prepared by treatment of formaldehyde with hydrogen sulfide.
Trithiane is a building block molecule in organic synthesis, being a masked source of formaldehyde. In one application, it is deprotonated with organolithium reagents to give the lithium derivative, which can be alkylated.
(CH2S)3 + RLi → (CH2S)2(CHLiS) + RH(CH2S)2(CHLiS) + R’Br → (CH2S)2(CHR’S) + LiBr(CH2S)2(CHR’S) + H2O → R’CHO + ….Trithiane is the dithioacetal of formaldehyde. Other dithioacetals undergo similar reactions to the above.
It is also a precursor to other organosulfur reagents. For example, chlorination in the presence of water affords the chloromethyl sulfonyl chloride:
(CH2S)3 + 9 Cl2 + 6 H2O → 3 ClCH2SO2Cl + 12 HClTrithianes
Trithiane is the parent of a class of heterocycles called trithianes. The species often arise from thiation of ketones and aldehydes. The incipient thioketones and thioaldehydes suffer trimerization. The reaction is reversed thermally.
