Formula C3H6O3 Melting point 64 °C Boiling point 114.5 °C Appearance White crystalline solid | Molar mass 90.08 g/mol Density 1.17 g/cm³ | |
 | ||
Related compounds | ||
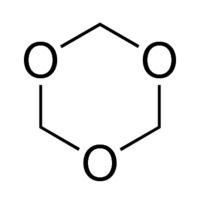
1,3,5-Trioxane, sometimes also called trioxane or trioxin, is a chemical compound with molecular formula C3H6O3. It is a white solid with a chloroform-like odor. It is a stable cyclic trimer of formaldehyde, and one of the three trioxane isomers; its molecular backbone consists of a six-membered ring with three carbon atoms alternating with three oxygen atoms. Thus, cyclotrimerization of formaldehyde affords 1,3,5-trioxane:
Contents
Production
Trioxane is produced by trimerization of formaldehyde using acid catalysts. The reaction is conducted in concentrated aqueous solution and the product is separated by solvent extraction. An idealized mechanism is shown below:
Uses
Trioxane is mainly consumed in the production of polyoxymethylene plastics, of which about 1M tons/y are produced. Other applications exploit its tendency to release formaldehyde. As such it is used as a binder in textiles, wood products, etc. Trioxane is combined with hexamine and compressed into solid bars to make hexamine fuel tablets, used by the military and outdoorsmen as a cooking fuel.
In the laboratory, trioxane is used as an anhydrous source of formaldehyde.
