 | ||
Terpenes terpenoids saponins and tocotrienols
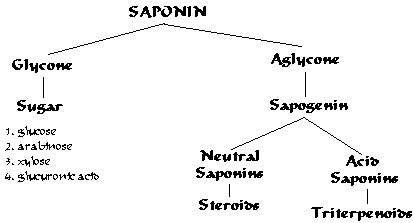
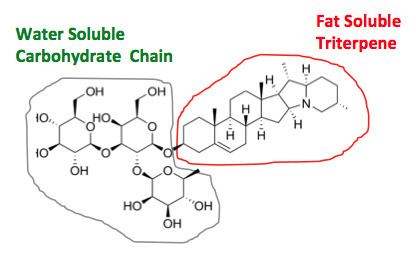
Saponins are a class of chemical compounds found in particular abundance in various plant species. More specifically, they are amphipathic glycosides grouped phenomenologically by the soap-like foaming they produce when shaken in aqueous solutions, and structurally by having one or more hydrophilic glycoside moieties combined with a lipophilic triterpene derivative.
Contents
- Terpenes terpenoids saponins and tocotrienols
- Are saponins in quinoa toxic
- Structural variety and biosynthesis
- Test
- Role in plant ecology and impact on animal foraging
- Ethnobotany
- Bioactivities
- Vaccine adjuvants
- Nutritional uses
- Use in animal feeding
- List of saponins
- References
Are saponins in quinoa toxic
Structural variety and biosynthesis
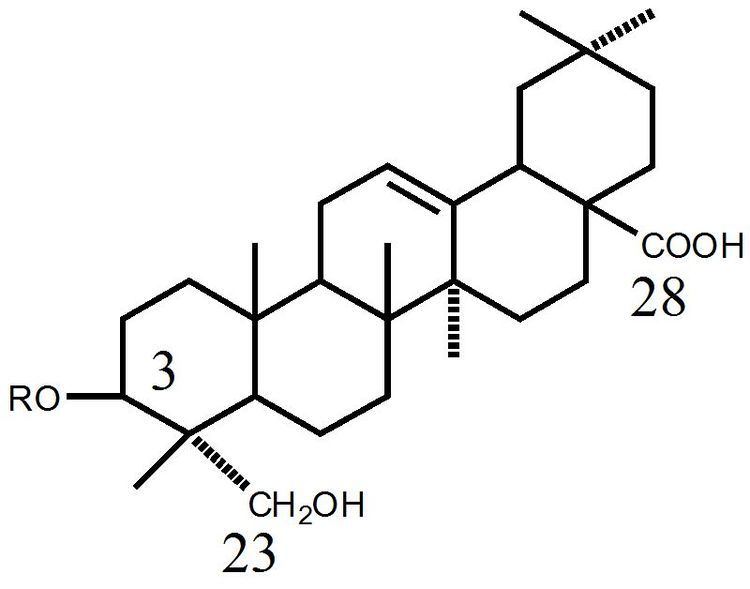
The aglycone (glycoside-free) portions of the saponins are termed sapogenins. The number of saccharide chains attached to the sapogenin/aglycone core can vary – giving rise to another dimension of nomenclature (monodesmosidic, bidesmosidic, etc.) – as can the length of each chain. A somewhat dated compilation has the range of saccharide chain lengths being 1–11, with the numbers 2-5 being the most frequent, and with both linear and branched chain saccharides being represented. Dietary monosaccharides such as D-glucose and D-galactose are among the most common components of the attached chains.
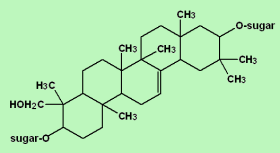
The lipophilic aglycone can be any one of a wide variety of polycyclic organic structures originating from the serial addition of 10-carbon (C10) terpene units to compose a C30 triterpene skeleton, often with subsequent alteration to produce a C27 steroidal skeleton. The subset of saponins that are steroidal have been termed saraponins; Aglycone derivatives can also incorporate nitrogen, so some saponins also present chemical and pharmacologic characteristics of alkaloid natural products. The figure at right above presents the structure of the alkaloid phytotoxin solanine, a monodesmosidic, branched-saccharide steroidal saponin. (The lipophilic steroidal structure is the series of connected six- and five-membered rings at the right of the structure, while the three oxygen-rich sugar rings are at left and below. Note the nitrogen atom inserted into the steroid skeleton at right.)
Test
Uses plant Gogo (bark) Entada phaseoloides as control. The positive result shows a honeycomb froth that is higher than 2 cm that persists for 10 minutes or longer.
Blood Agar Media (BAM): Is an agar cup semi-quantitative method that shows positive result of hemolytic halos.
Role in plant ecology and impact on animal foraging

In plants, saponins may serve as anti-feedants, and to protect the plant against microbes and fungi. Some plant saponins (e.g. from oat and spinach) may enhance nutrient absorption and aid in animal digestion. However, saponins are often bitter to taste, and so can reduce plant palatability (e.g., in livestock feeds), or even imbue them with life-threatening animal toxicity. Some saponins are toxic to cold-blooded organisms and insects at particular concentrations. Further research is needed to define the roles of these natural products in their host organisms, which have been described as "poorly understood" to date.
Ethnobotany
Most saponins, which readily dissolve in water, are poisonous to fish. Therefore, in ethnobotany, they are primarily known for their use by indigenous people in obtaining aquatic food sources.
Since prehistoric times, cultures throughout the world have used piscicidal (fish-killing) plants, mostly those containing saponins, for fishing.
Although prohibited by law, fish poison plants are still widely used by indigenous tribes in Guyana.
On the Indian Subcontinent, the Gond tribes are known for their use of plant extracts in poison fishing.
Many of California's Native American tribes traditionally used soaproot, (genus Chlorogalum) and/or the root of various yucca species, which contain saponin, as a fish poison. They would pulverize the roots, mixing in water to create a foam, and then add the suds to a stream. This would kill or incapacitate the fish, which could be gathered easily from the surface of the water. Among the tribes using this technique were the Lassik, the Luiseño, and the Mattole.
Bioactivities
One research use of the saponin class of natural products involves their complexation with cholesterol to form pores in cell membrane bilayers, e.g., in red cell (erythrocyte) membranes, where complexation leads to red cell lysis (hemolysis) on intravenous injection. In addition, the amphipathic nature of the class gives them activity as surfactants that can be used to enhance penetration of macromolecules such as proteins through cell membranes.
Saponins from the Gypsophila paniculata (baby’s breath) plant have been shown to significantly augment the cytotoxicity of immunotoxins and other targeted toxins directed against human cancer cells. The research groups of Professor Hendrik Fuchs (Charité University, Berlin, Germany) and Dr David Flavell (Southampton General Hospital, United Kingdom) are working together toward the development of Gypsophila saponins for use in combination with immunotoxins or other targeted toxins for patients with leukaemia, lymphoma and other cancers.
Vaccine adjuvants
Saponins have also been used as adjuvants in vaccines, e.g. Quil A component QS-21, isolated from the bark of Quillaja saponaria Molina, to stimulate both the Th1 immune response and the production of cytotoxic T-lymphocytes (CTLs) against exogenous antigens. This makes them ideal for use in subunit vaccines and vaccines directed against intracellular pathogens as well as for therapeutic cancer vaccines but with the aforementioned side-effects of hemolysis.
In their use as adjuvants in the production of vaccines, toxicity associated with sterol complexation remains a major issue for attention.
Nutritional uses
Saponins are being promoted commercially as dietary supplements and food ingredients. There is evidence of the presence of saponins in traditional medicine preparations from licorice, where oral administrations might be expected to lead to hydrolysis of glycoside from terpenoid (and obviation of any toxicity associated with the intact molecule).
But as is often the case with wide-ranging commercial therapeutic claims for natural products:
While such statements require constant review (and despite the myriad web claims to the contrary), it appears that there are very limited US, EU, etc. agency-approved roles for saponins in human therapy. Therapeutic benefit is a result of careful administration of an appropriate dose. Very great care needs to be exercised in evaluating or acting on specific claims of therapeutic benefit from ingesting saponin-type and other natural products.
Use in animal feeding
Saponins are used widely for their effects on ammonia emissions in animal feeding. The mode of action seems to be an inhibition of the urease enzyme, which splits up excreted urea in feces into ammonia and carbon dioxide. Animal trials have shown that a reduced ammonia level in farming operations causes less damages to the respiratory tract of animals, and may help to make them less vulnerable to diseases.
